Iron »
PDB 6ux0-6vk7 »
6uyk »
Iron in PDB 6uyk: Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
Enzymatic activity of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
All present enzymatic activity of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.:
1.3.7.7;
1.3.7.7;
Protein crystallography data
The structure of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form., PDB code: 6uyk
was solved by
J.P.Bacik,
S.M.S.Imran,
M.B.Watkins,
E.Corless,
E.Antony,
N.Ando,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.71 / 2.60 |
| Space group | I 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 92.513, 100.918, 117.789, 90.00, 99.27, 90.00 |
| R / Rfree (%) | 23.1 / 27.7 |
Other elements in 6uyk:
The structure of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
(pdb code 6uyk). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 8 binding sites of Iron where determined in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form., PDB code: 6uyk:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Iron where determined in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form., PDB code: 6uyk:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Iron binding site 1 out of 8 in 6uyk
Go back to
Iron binding site 1 out
of 8 in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
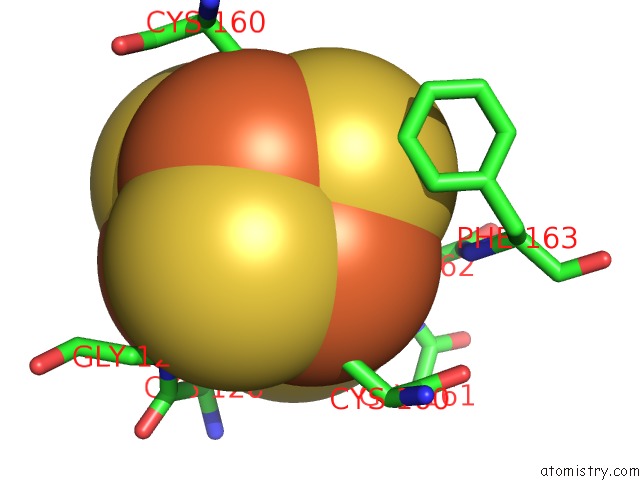
Mono view
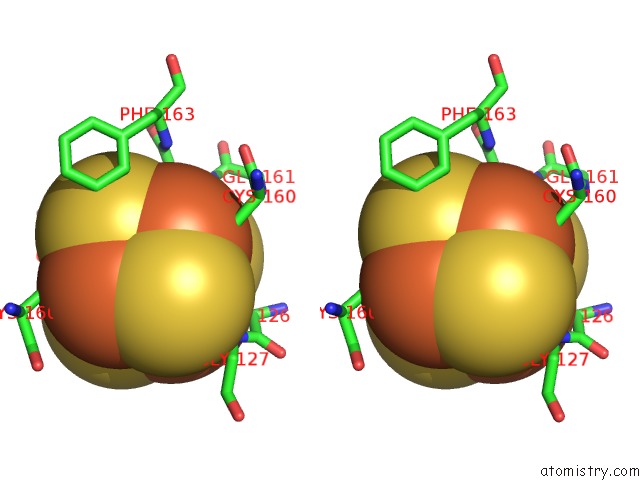
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. within 5.0Å range:
|
Iron binding site 2 out of 8 in 6uyk
Go back to
Iron binding site 2 out
of 8 in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
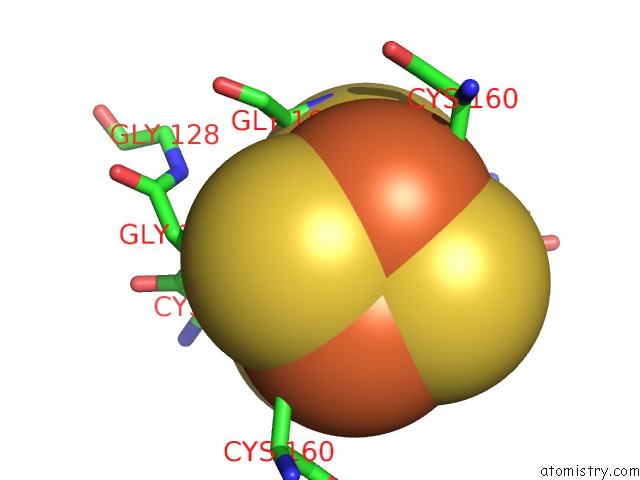
Mono view
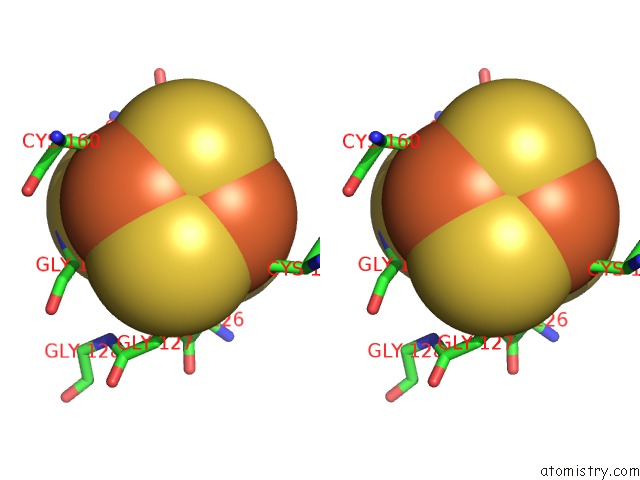
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. within 5.0Å range:
|
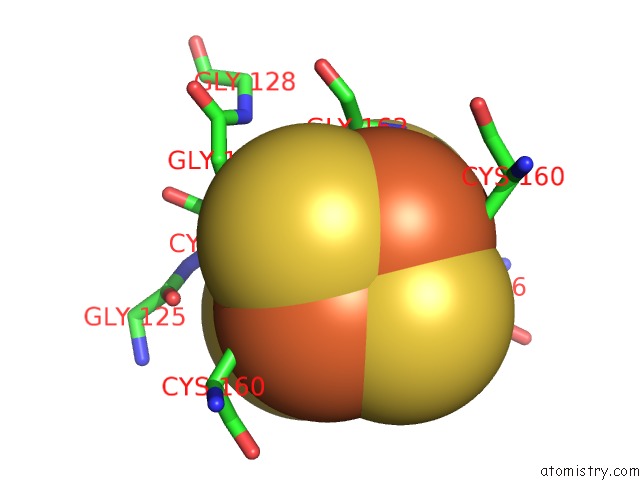
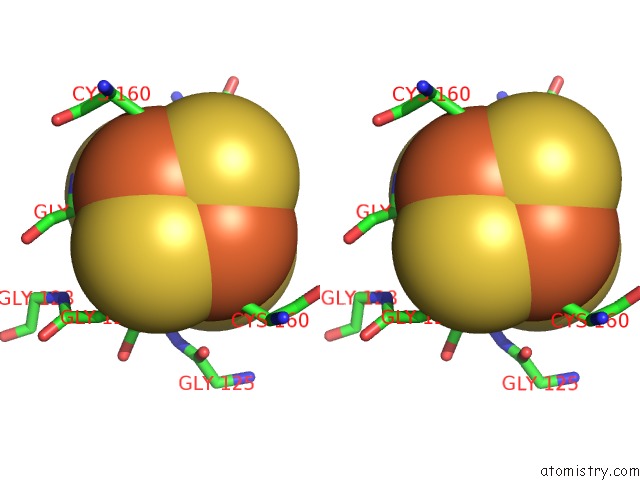
Iron binding site 3 out of 8 in 6uyk
Go back to
Iron binding site 3 out
of 8 in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. within 5.0Å range:
|
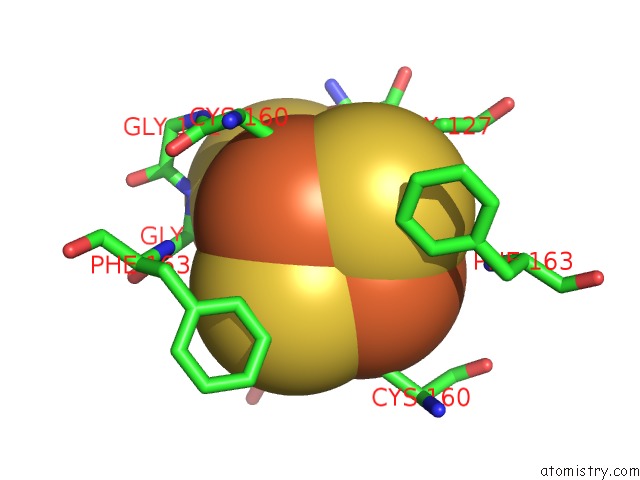
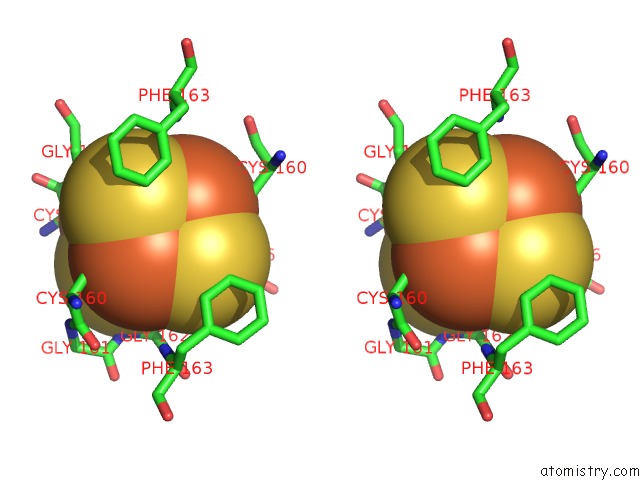
Iron binding site 4 out of 8 in 6uyk
Go back to
Iron binding site 4 out
of 8 in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. within 5.0Å range:
|
Iron binding site 5 out of 8 in 6uyk
Go back to
Iron binding site 5 out
of 8 in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
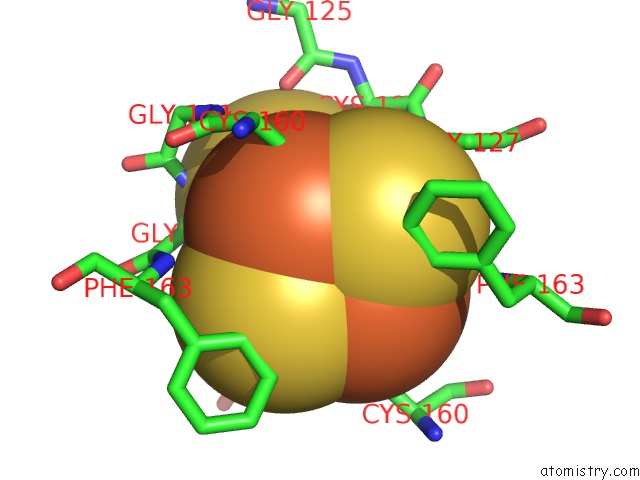
Mono view
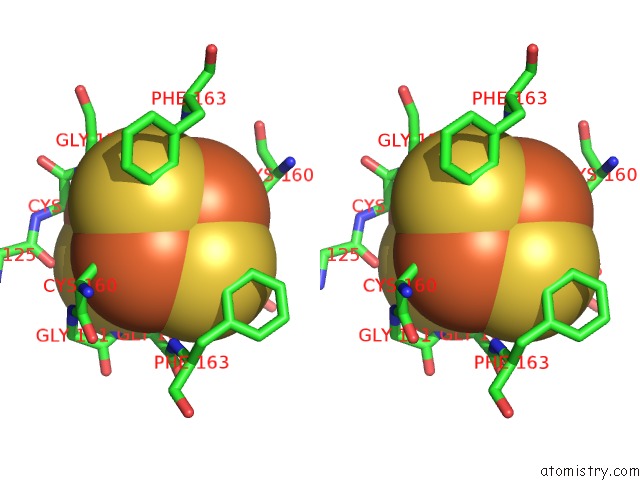
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. within 5.0Å range:
|
Iron binding site 6 out of 8 in 6uyk
Go back to
Iron binding site 6 out
of 8 in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
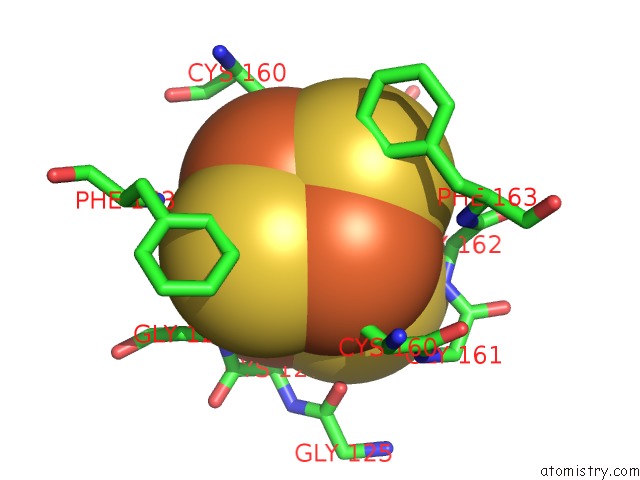
Mono view
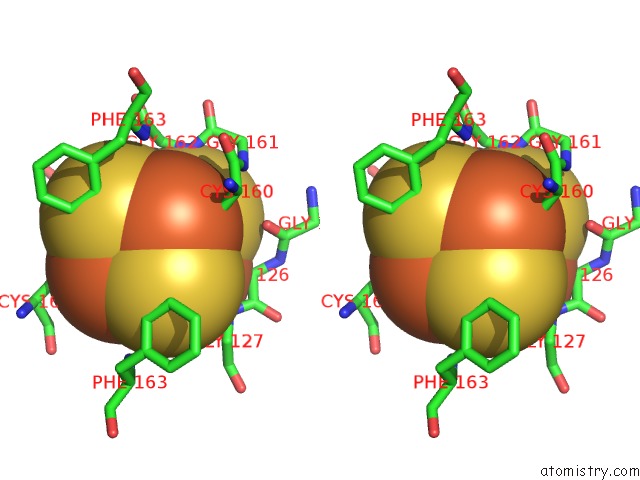
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. within 5.0Å range:
|
Iron binding site 7 out of 8 in 6uyk
Go back to
Iron binding site 7 out
of 8 in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
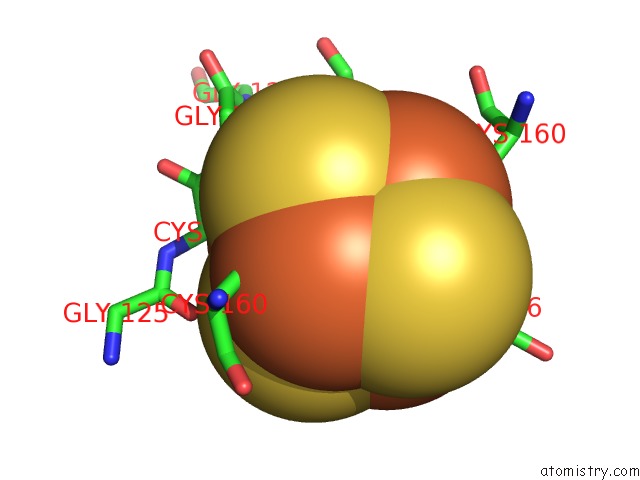
Mono view
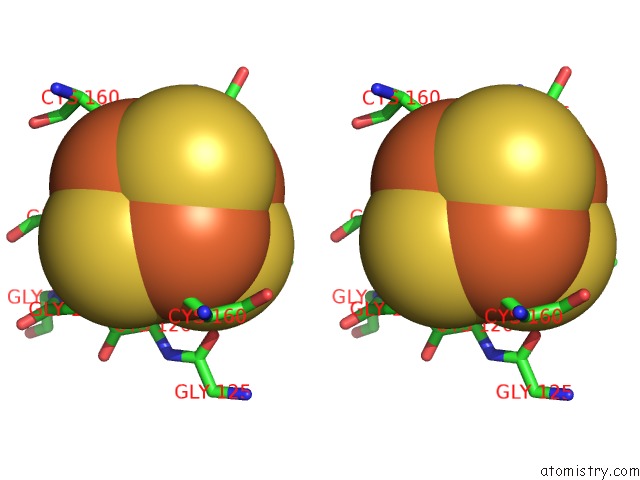
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. within 5.0Å range:
|
Iron binding site 8 out of 8 in 6uyk
Go back to
Iron binding site 8 out
of 8 in the Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form.
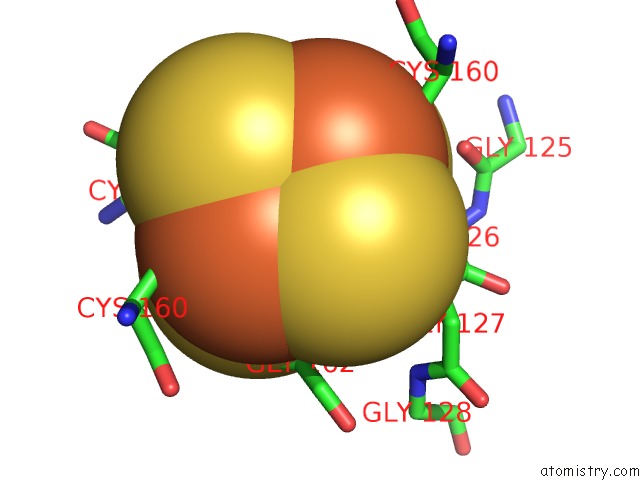
Mono view
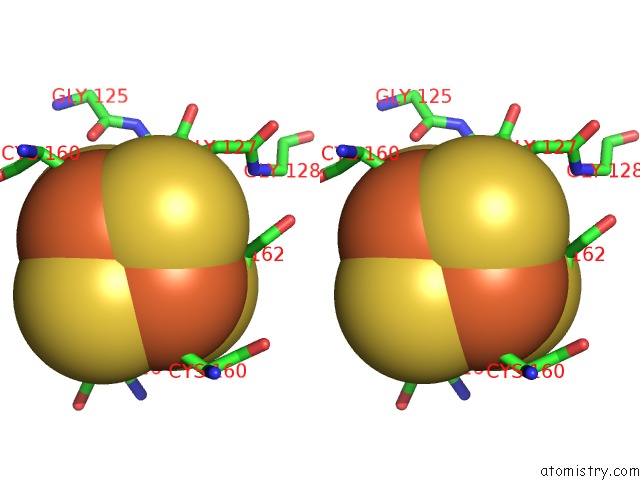
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Dark-Operative Protochlorophyllide Oxidoreductase in the Nucleotide- Free Form. within 5.0Å range:
|
Reference:
E.I.Corless,
S.M.Saad Imran,
M.B.Watkins,
J.P.Bacik,
J.Mattice,
A.Patterson,
K.Danyal,
M.Soffe,
R.Kitelinger,
L.C.Seefeldt,
S.S.Origanti,
B.Bennett,
B.Bothner,
N.Ando,
E.Antony.
The Flexible N-Terminus of Bchl Autoinhibits Activity Through Interaction with Its [4FE-4S] Cluster and Relieved Upon Atp Binding. J.Biol.Chem. 2020.
ISSN: ESSN 1083-351X
PubMed: 33219127
DOI: 10.1074/JBC.RA120.016278
Page generated: Wed Aug 7 12:57:13 2024
ISSN: ESSN 1083-351X
PubMed: 33219127
DOI: 10.1074/JBC.RA120.016278
Last articles
Cl in 8BZ9Cl in 8BZB
Cl in 8BZ8
Cl in 8BZ7
Cl in 8BZ6
Cl in 8BYH
Cl in 8BZ4
Cl in 8BYY
Cl in 8BYG
Cl in 8BYO