Iron »
PDB 6vk8-6wnb »
6vxt »
Iron in PDB 6vxt: Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
Enzymatic activity of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
All present enzymatic activity of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii:
1.18.6.1;
1.18.6.1;
Protein crystallography data
The structure of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii, PDB code: 6vxt
was solved by
W.Kang,
Y.Hu,
M.W.Ribbe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.71 / 1.74 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 163.535, 203.435, 83.857, 90.00, 103.95, 90.00 |
| R / Rfree (%) | 17.2 / 20.3 |
Other elements in 6vxt:
The structure of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii also contains other interesting chemical elements:
| Molybdenum | (Mo) | 10 atoms |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20; Page 3, Binding sites: 21 - 30; Page 4, Binding sites: 31 - 32;Binding sites:
The binding sites of Iron atom in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii (pdb code 6vxt). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 32 binding sites of Iron where determined in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii, PDB code: 6vxt:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 32 in 6vxt
Go back to
Iron binding site 1 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
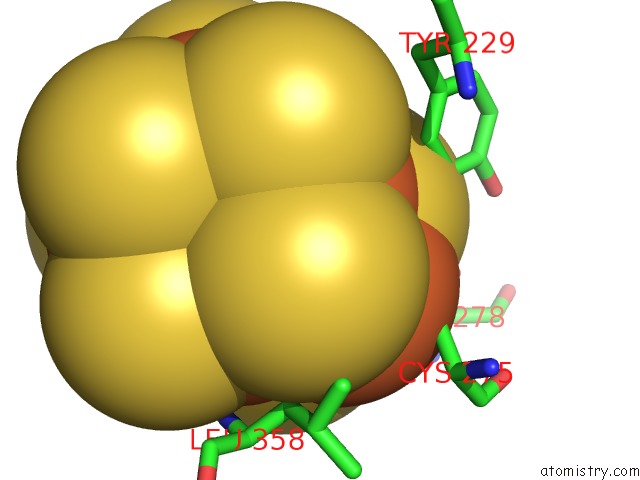
Mono view
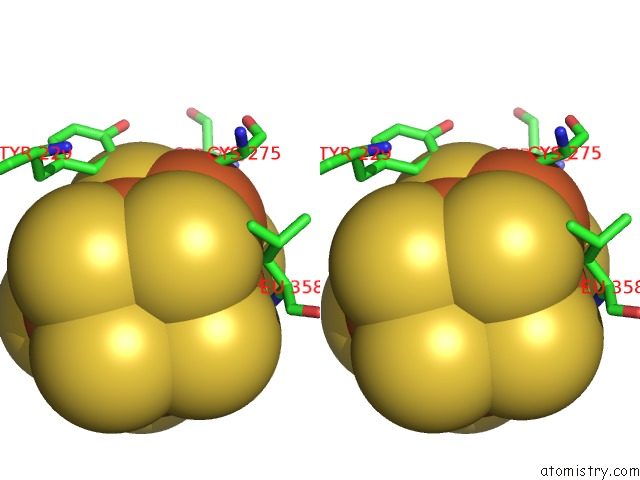
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 2 out of 32 in 6vxt
Go back to
Iron binding site 2 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
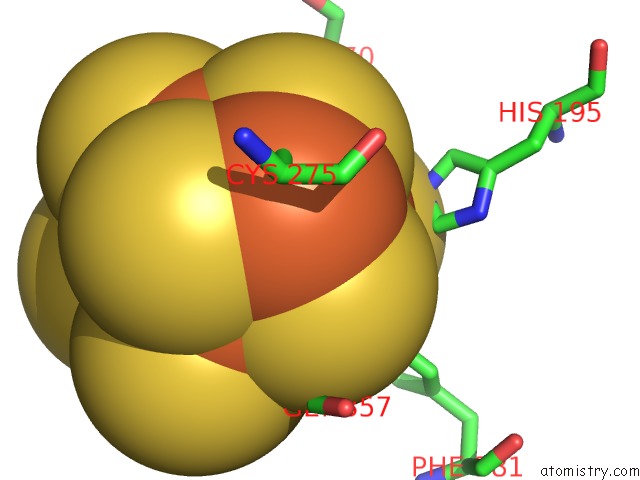
Mono view
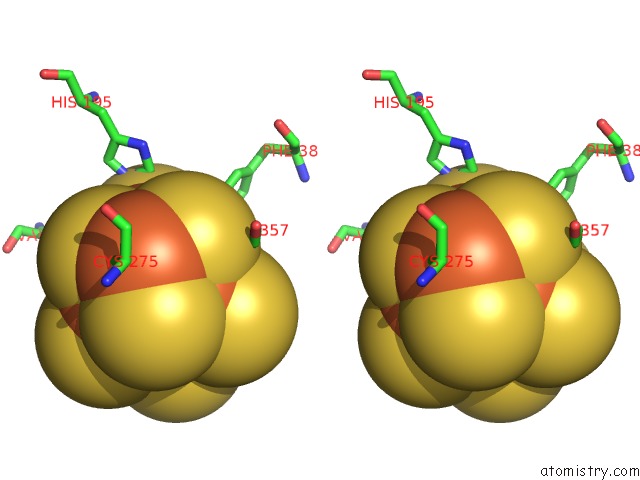
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 3 out of 32 in 6vxt
Go back to
Iron binding site 3 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
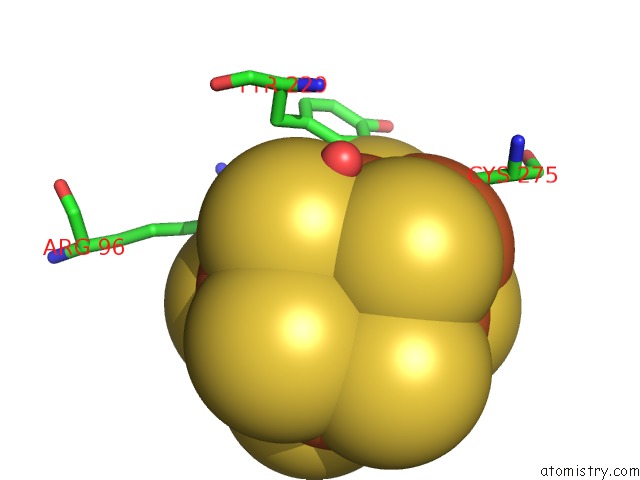
Mono view
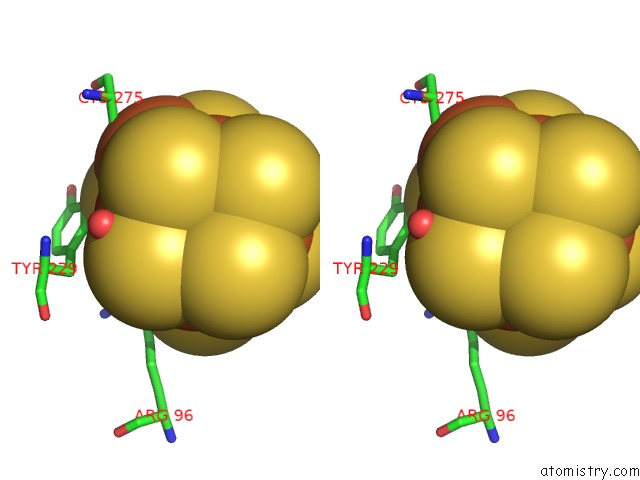
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 4 out of 32 in 6vxt
Go back to
Iron binding site 4 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
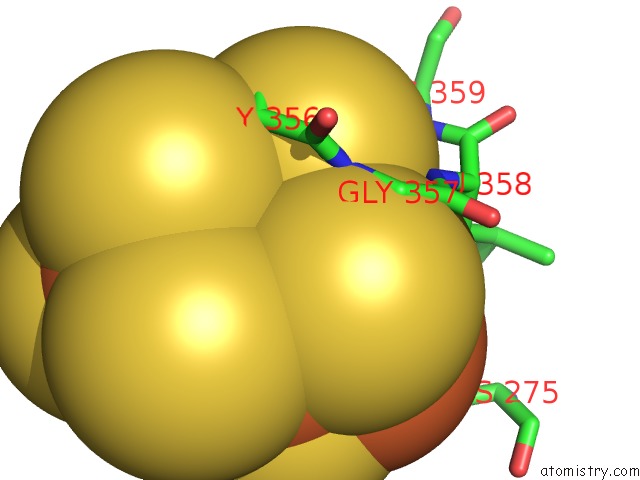
Mono view
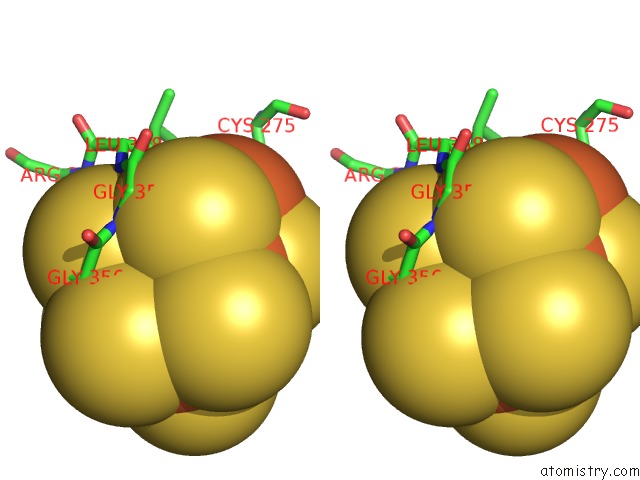
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 5 out of 32 in 6vxt
Go back to
Iron binding site 5 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 6 out of 32 in 6vxt
Go back to
Iron binding site 6 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 7 out of 32 in 6vxt
Go back to
Iron binding site 7 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
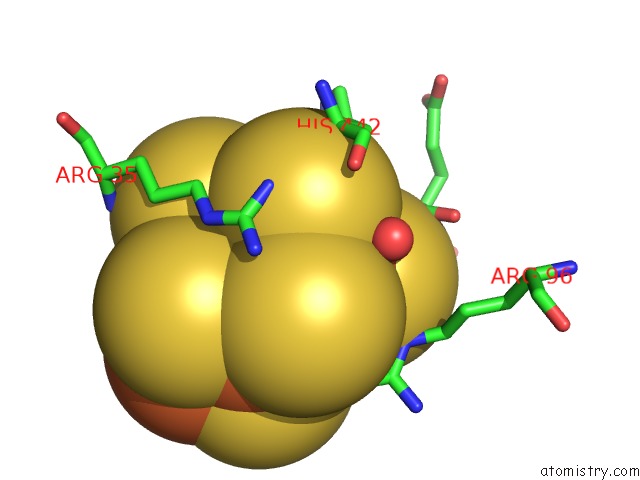
Mono view
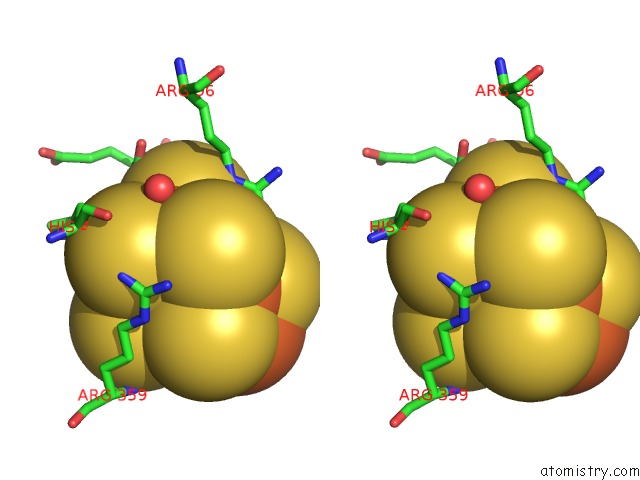
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 8 out of 32 in 6vxt
Go back to
Iron binding site 8 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
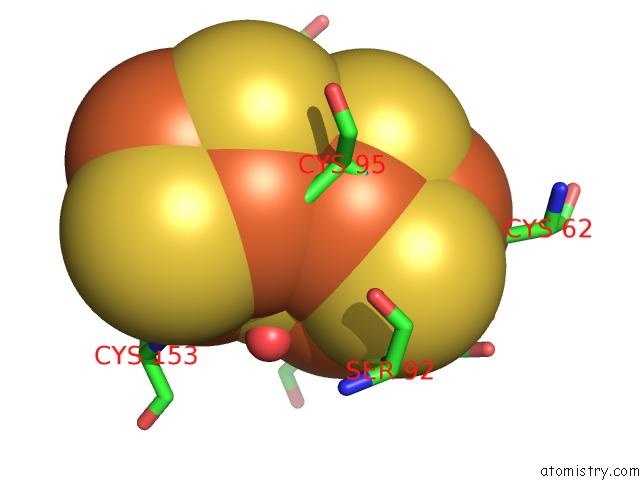
Mono view
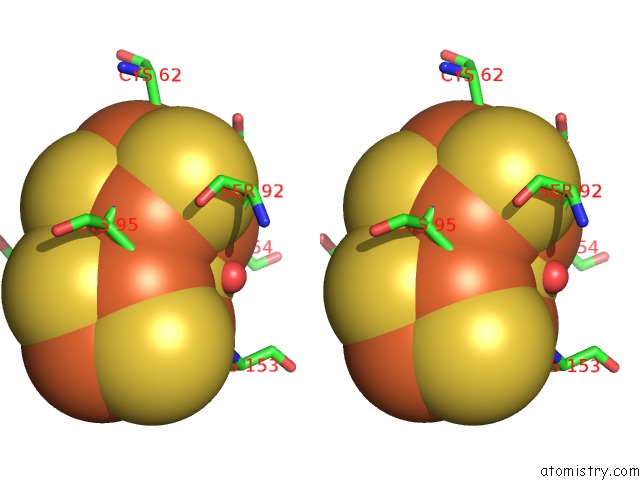
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 9 out of 32 in 6vxt
Go back to
Iron binding site 9 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
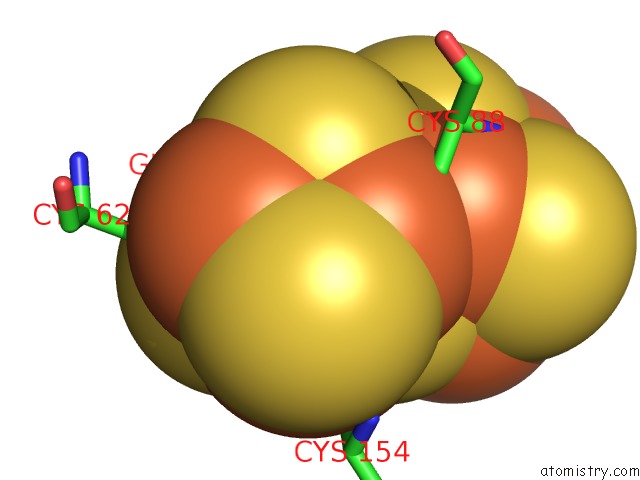
Mono view
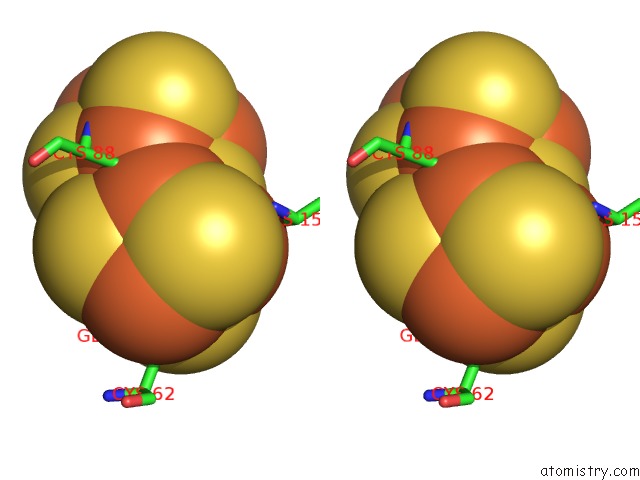
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 10 out of 32 in 6vxt
Go back to
Iron binding site 10 out
of 32 in the Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii
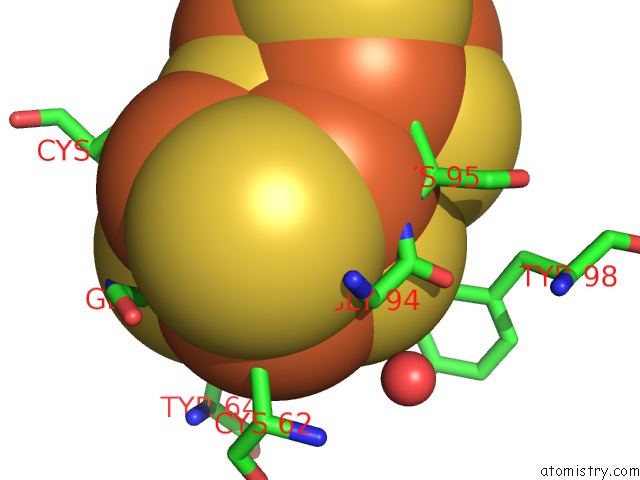
Mono view
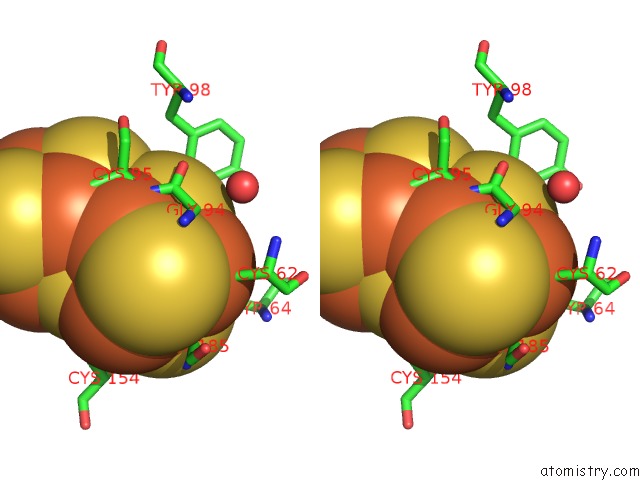
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Activated Nitrogenase Mofe-Protein From Azotobacter Vinelandii within 5.0Å range:
|
Reference:
W.Kang,
C.C.Lee,
A.J.Jasniewski,
M.W.Ribbe,
Y.Hu.
Structural Evidence For A Dynamic Metallocofactor During N Reduction By Mo-Nitrogenase Science 2020.
ISSN: ESSN 1095-9203
DOI: 10.1126/SCIENCE.AAZ6748
Page generated: Wed Aug 7 13:39:21 2024
ISSN: ESSN 1095-9203
DOI: 10.1126/SCIENCE.AAZ6748
Last articles
Cl in 8CP8Cl in 8CTB
Cl in 8CSC
Cl in 8CRM
Cl in 8CRH
Cl in 8CRK
Cl in 8CQP
Cl in 8CRI
Cl in 8CRC
Cl in 8CQT