Iron »
PDB 6zag-6zkg »
6zh5 »
Iron in PDB 6zh5: Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 16;Binding sites:
The binding sites of Iron atom in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor (pdb code 6zh5). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 16 binding sites of Iron where determined in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor, PDB code: 6zh5:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 16 in 6zh5
Go back to
Iron binding site 1 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
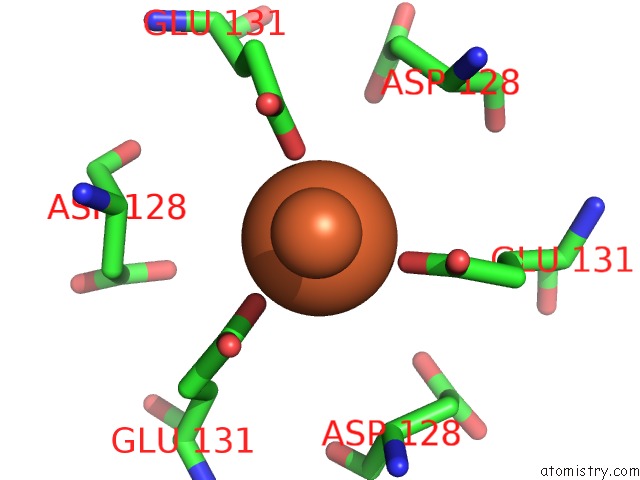
Mono view
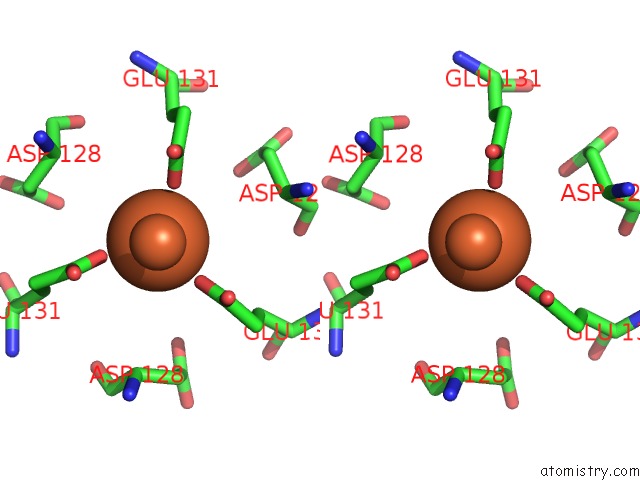
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
Iron binding site 2 out of 16 in 6zh5
Go back to
Iron binding site 2 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
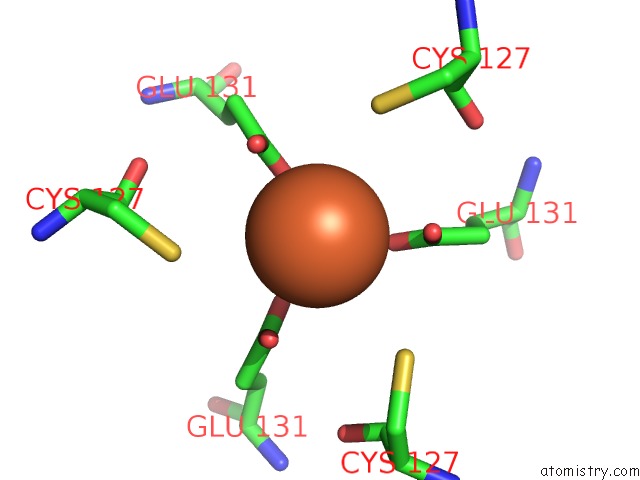
Mono view
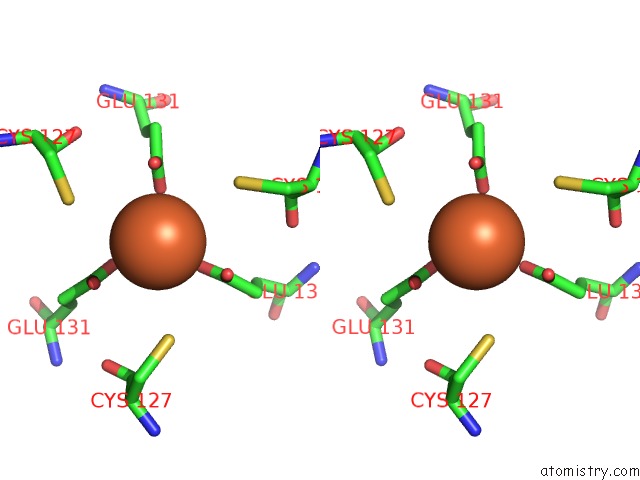
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
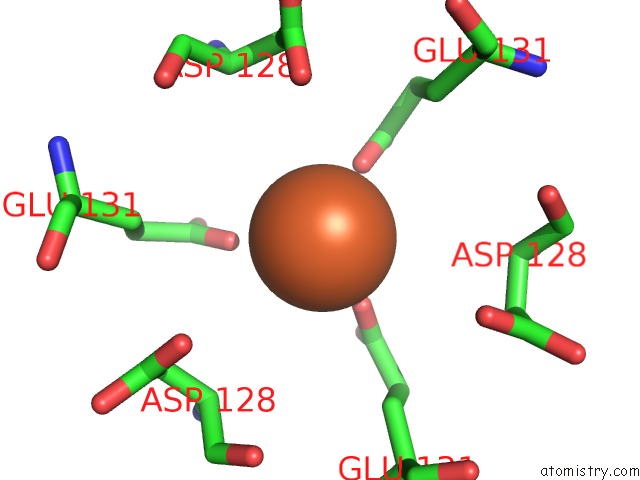
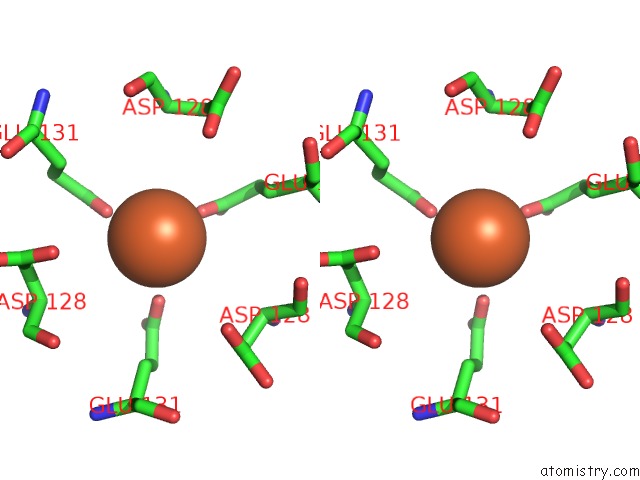
Iron binding site 3 out of 16 in 6zh5
Go back to
Iron binding site 3 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
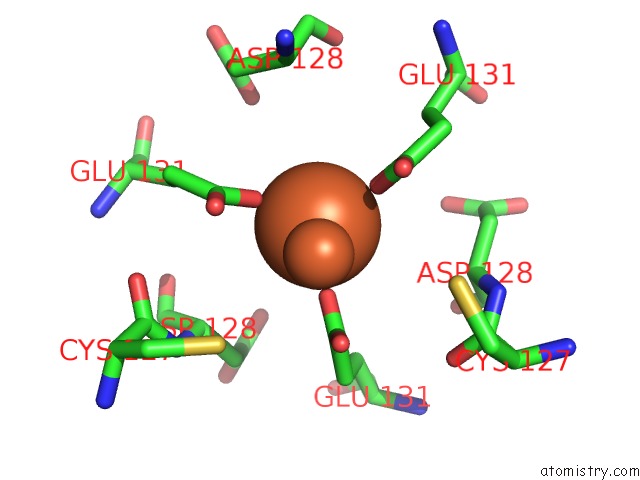
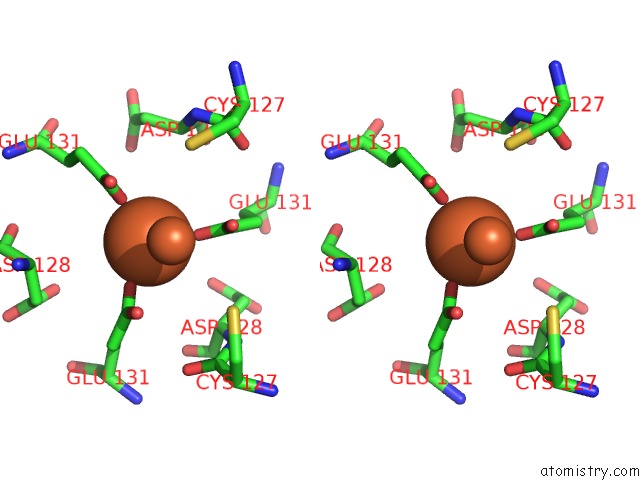
Iron binding site 4 out of 16 in 6zh5
Go back to
Iron binding site 4 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
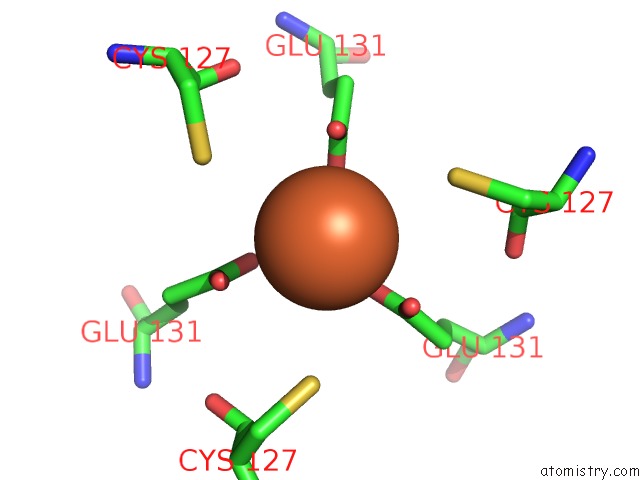
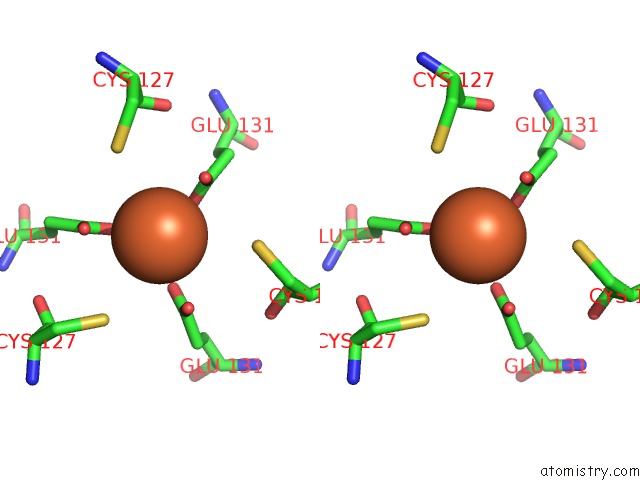
Iron binding site 5 out of 16 in 6zh5
Go back to
Iron binding site 5 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
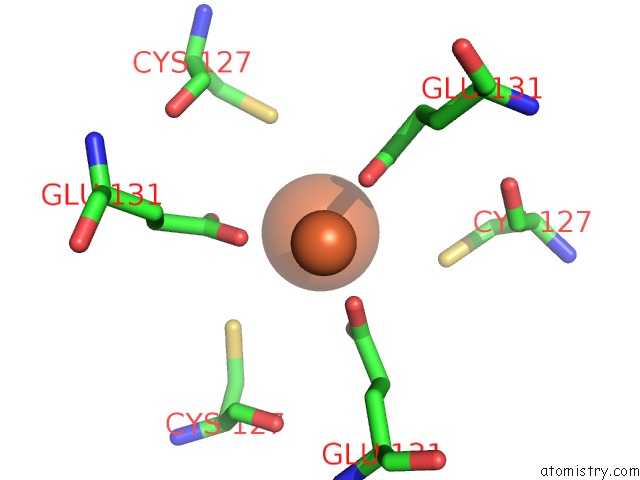
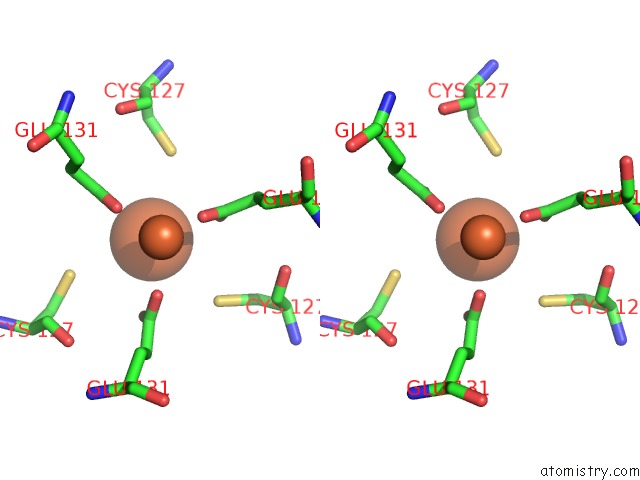
Iron binding site 6 out of 16 in 6zh5
Go back to
Iron binding site 6 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
Iron binding site 7 out of 16 in 6zh5
Go back to
Iron binding site 7 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
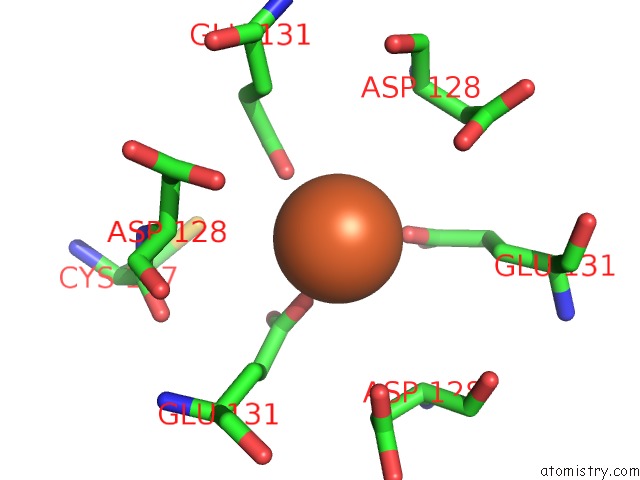
Mono view
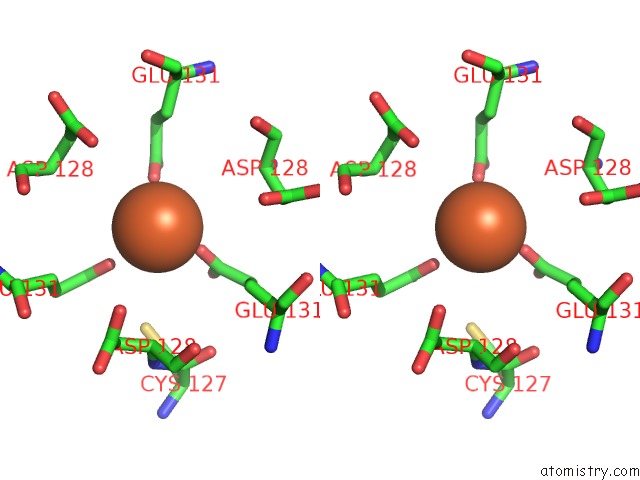
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
Iron binding site 8 out of 16 in 6zh5
Go back to
Iron binding site 8 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
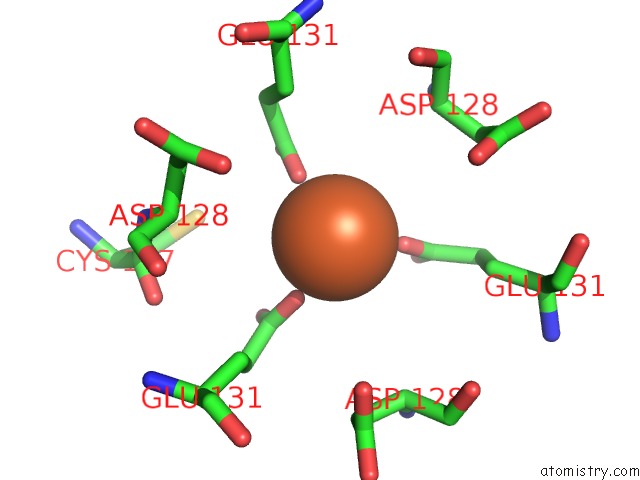
Mono view
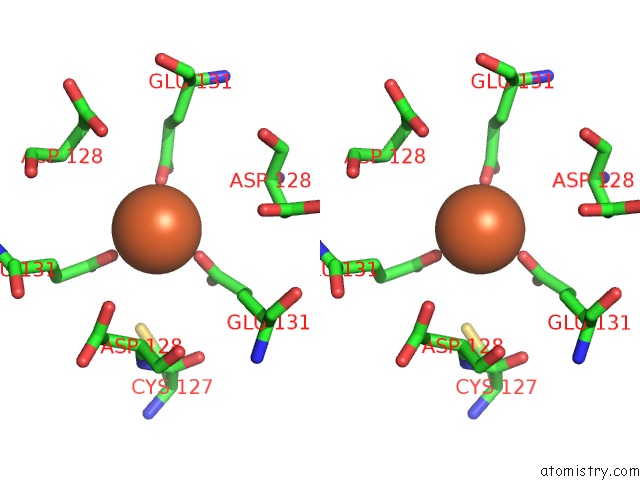
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
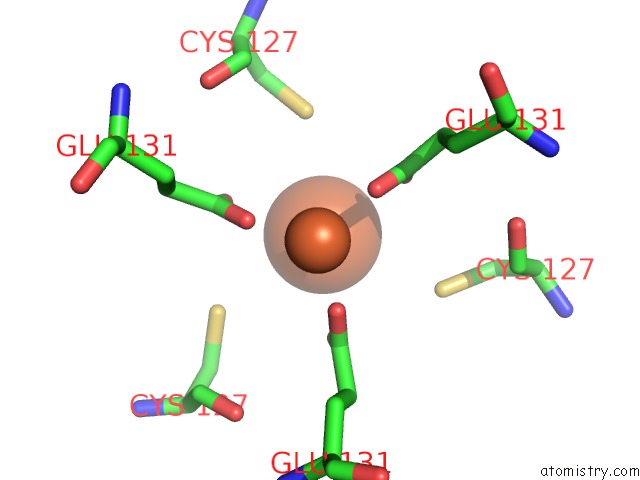
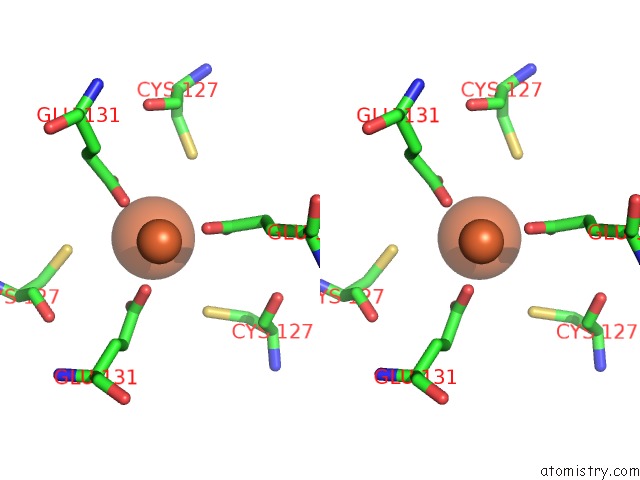
Iron binding site 9 out of 16 in 6zh5
Go back to
Iron binding site 9 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
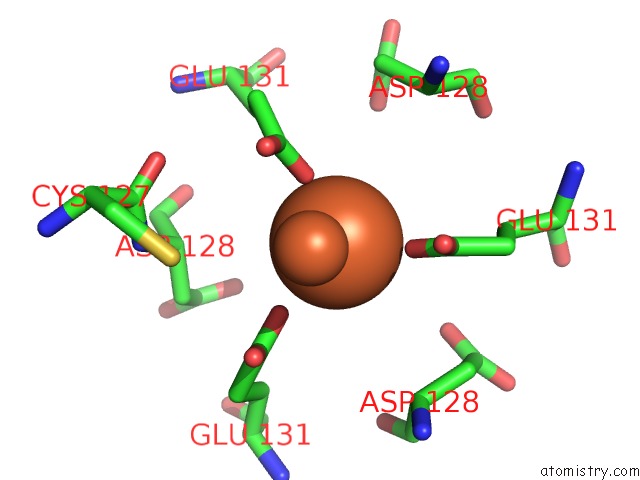
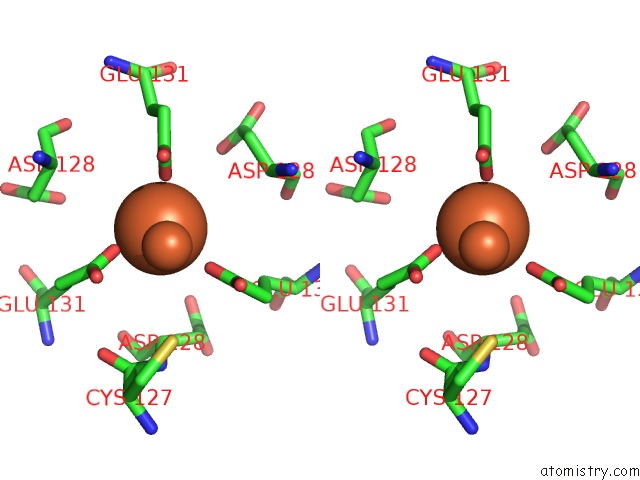
Iron binding site 10 out of 16 in 6zh5
Go back to
Iron binding site 10 out
of 16 in the Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Folding of An Iron Binding Peptide in Response to Sedimentation Is Resolved Using Ferritin As A Nano-Reactor within 5.0Å range:
|
Reference:
G.Davidov,
G.Abelya,
R.Zalk,
B.Izbicki,
S.Shaibi,
L.Spektor,
D.Shagidov,
E.G.Meyron-Holtz,
R.Zarivach,
G.A.Frank.
Folding of An Intrinsically Disordered Iron-Binding Peptide in Response to Sedimentation Revealed By Cryo-Em. J.Am.Chem.Soc. V. 142 19551 2020.
ISSN: ESSN 1520-5126
PubMed: 33166133
DOI: 10.1021/JACS.0C07565
Page generated: Wed Aug 7 18:24:28 2024
ISSN: ESSN 1520-5126
PubMed: 33166133
DOI: 10.1021/JACS.0C07565
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF