Iron »
PDB 7czl-7dgj »
7deg »
Iron in PDB 7deg: Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism
Other elements in 7deg:
The structure of Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism also contains other interesting chemical elements:
| Copper | (Cu) | 6 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism
(pdb code 7deg). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism, PDB code: 7deg:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism, PDB code: 7deg:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 7deg
Go back to
Iron binding site 1 out
of 4 in the Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism
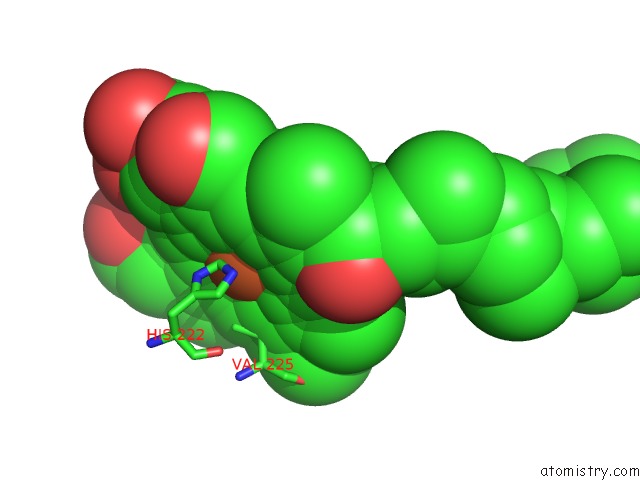
Mono view
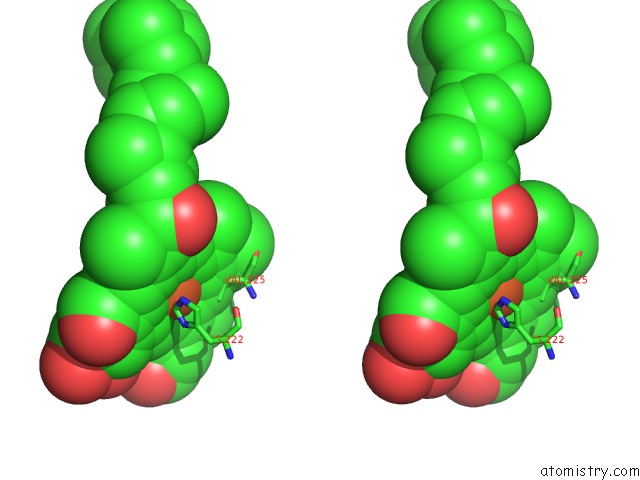
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism within 5.0Å range:
|
Iron binding site 2 out of 4 in 7deg
Go back to
Iron binding site 2 out
of 4 in the Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism
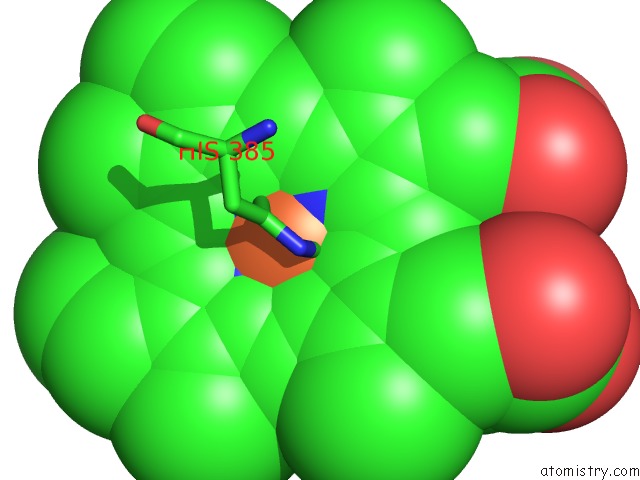
Mono view
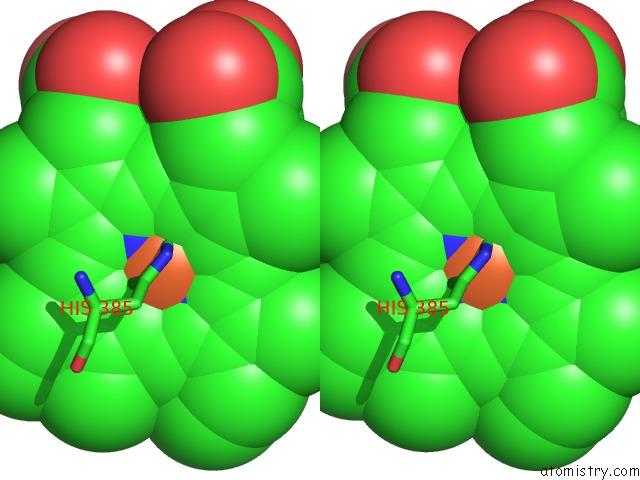
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism within 5.0Å range:
|
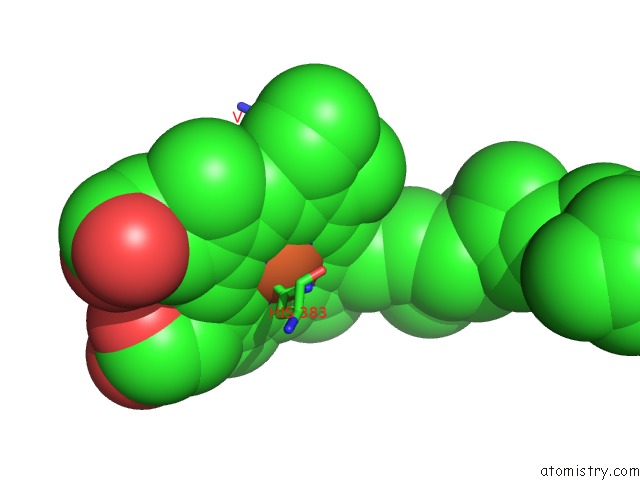
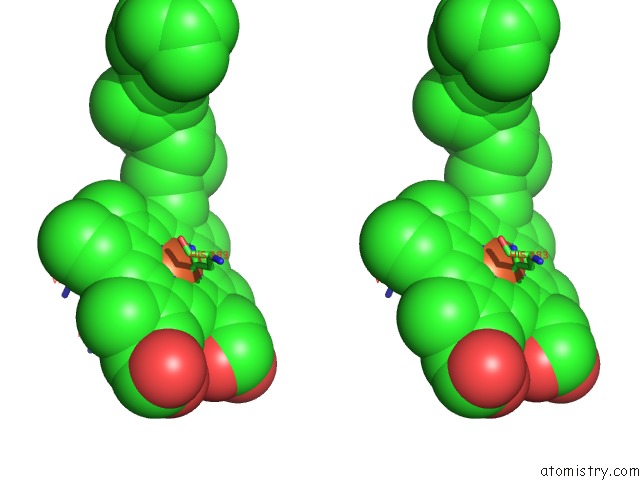
Iron binding site 3 out of 4 in 7deg
Go back to
Iron binding site 3 out
of 4 in the Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism within 5.0Å range:
|
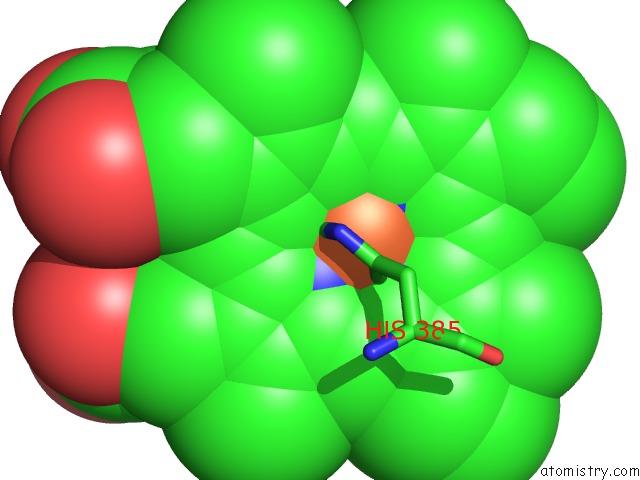
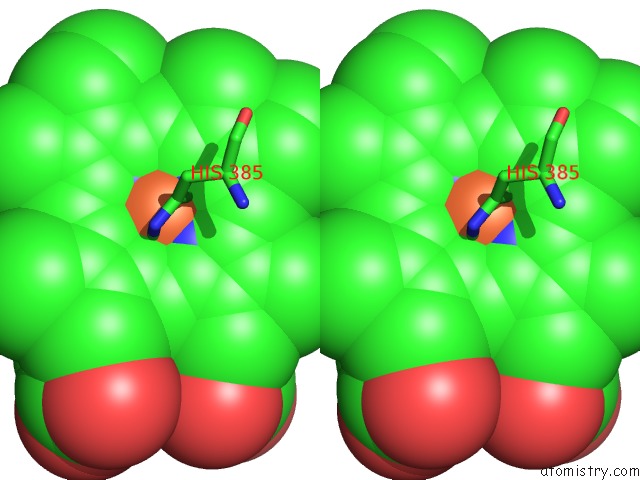
Iron binding site 4 out of 4 in 7deg
Go back to
Iron binding site 4 out
of 4 in the Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cryo-Em Structure of A Heme-Copper Terminal Oxidase Dimer Provides Insights Into Its Catalytic Mechanism within 5.0Å range:
|
Reference:
G.Zhu,
H.Zeng,
S.Zhang,
J.Juli,
L.Tai,
D.Zhang,
X.Pang,
Y.Zhang,
S.M.Lam,
Y.Zhu,
G.Peng,
H.Michel,
F.Sun.
The Unusual Homodimer of A Heme-Copper Terminal Oxidase Allows Itself to Utilize Two Electron Donors. Angew.Chem.Int.Ed.Engl. V. 60 13323 2021.
ISSN: ESSN 1521-3773
PubMed: 33665933
DOI: 10.1002/ANIE.202016785
Page generated: Thu Aug 8 03:49:24 2024
ISSN: ESSN 1521-3773
PubMed: 33665933
DOI: 10.1002/ANIE.202016785
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW