Iron »
PDB 7orc-7p7k »
7p6l »
Iron in PDB 7p6l: Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid
Enzymatic activity of Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid
All present enzymatic activity of Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid:
1.14.14.1; 1.6.2.4;
1.14.14.1; 1.6.2.4;
Protein crystallography data
The structure of Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid, PDB code: 7p6l
was solved by
D.J.Opperman,
J.C.Aschenbrenner,
C.Tolmie,
A.C.Ebrecht,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 61.43 / 2.33 |
| Space group | P 42 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 171.49, 171.49, 175.647, 90, 90, 90 |
| R / Rfree (%) | 20.3 / 23.4 |
Iron Binding Sites:
The binding sites of Iron atom in the Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid
(pdb code 7p6l). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid, PDB code: 7p6l:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid, PDB code: 7p6l:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 7p6l
Go back to
Iron binding site 1 out
of 4 in the Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid
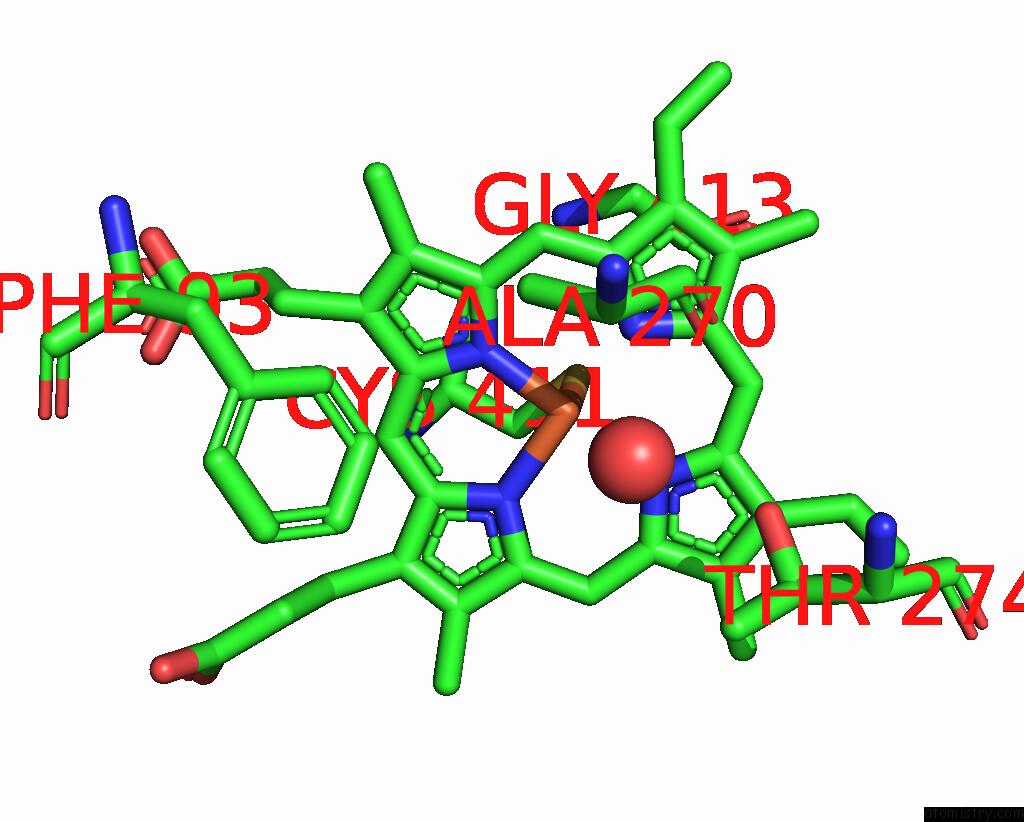
Mono view
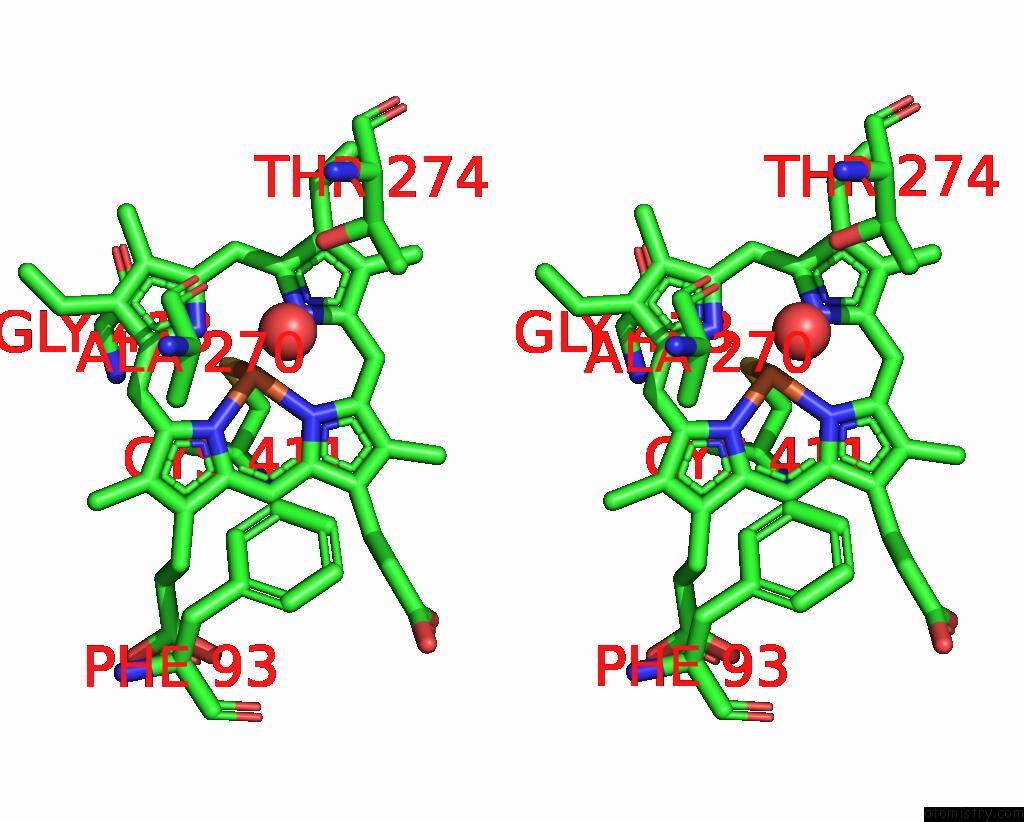
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid within 5.0Å range:
|
Iron binding site 2 out of 4 in 7p6l
Go back to
Iron binding site 2 out
of 4 in the Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid
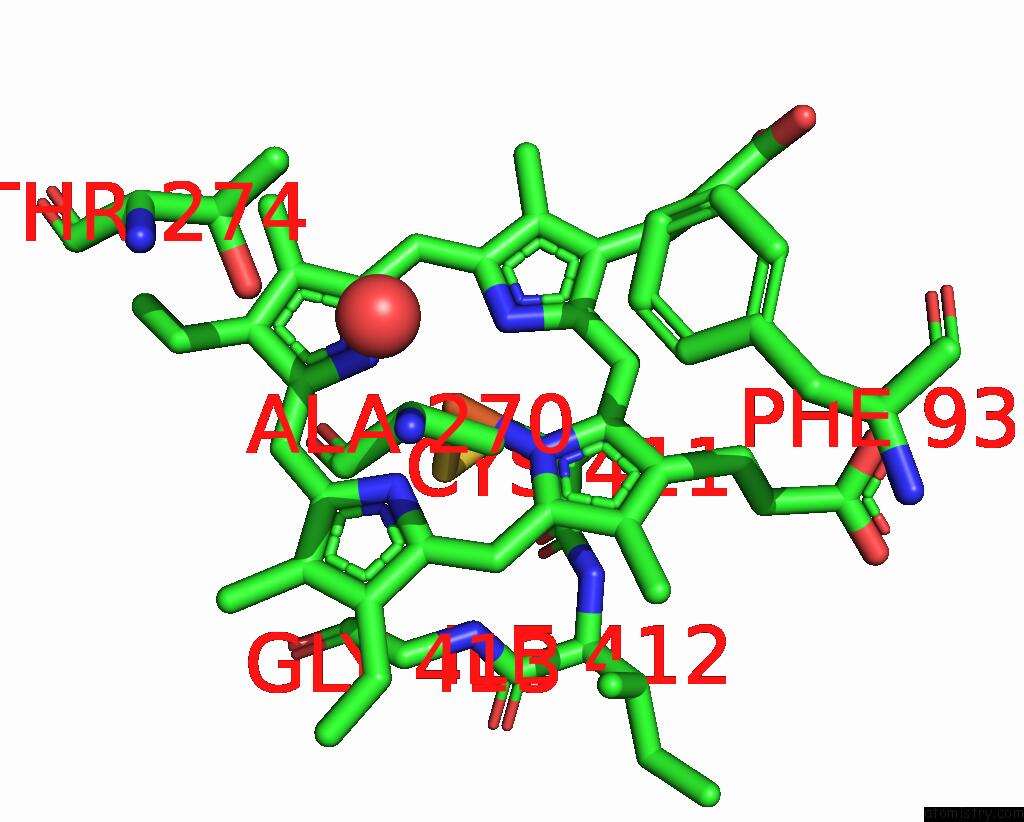
Mono view
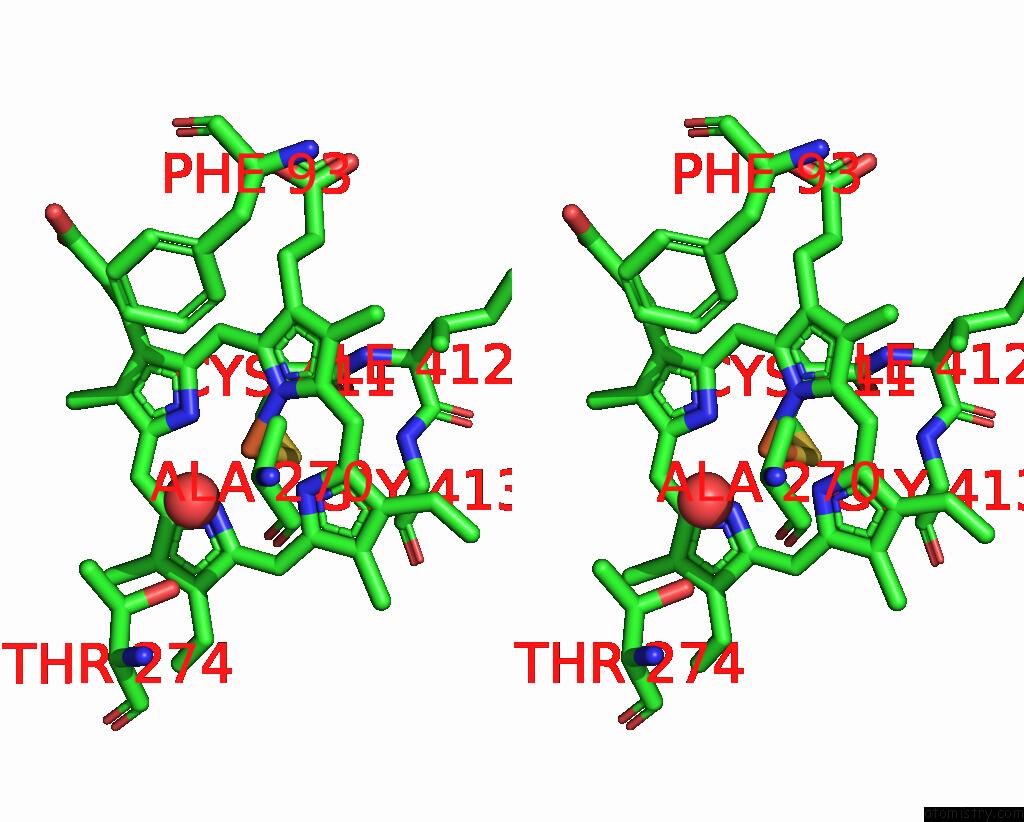
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid within 5.0Å range:
|
Iron binding site 3 out of 4 in 7p6l
Go back to
Iron binding site 3 out
of 4 in the Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid
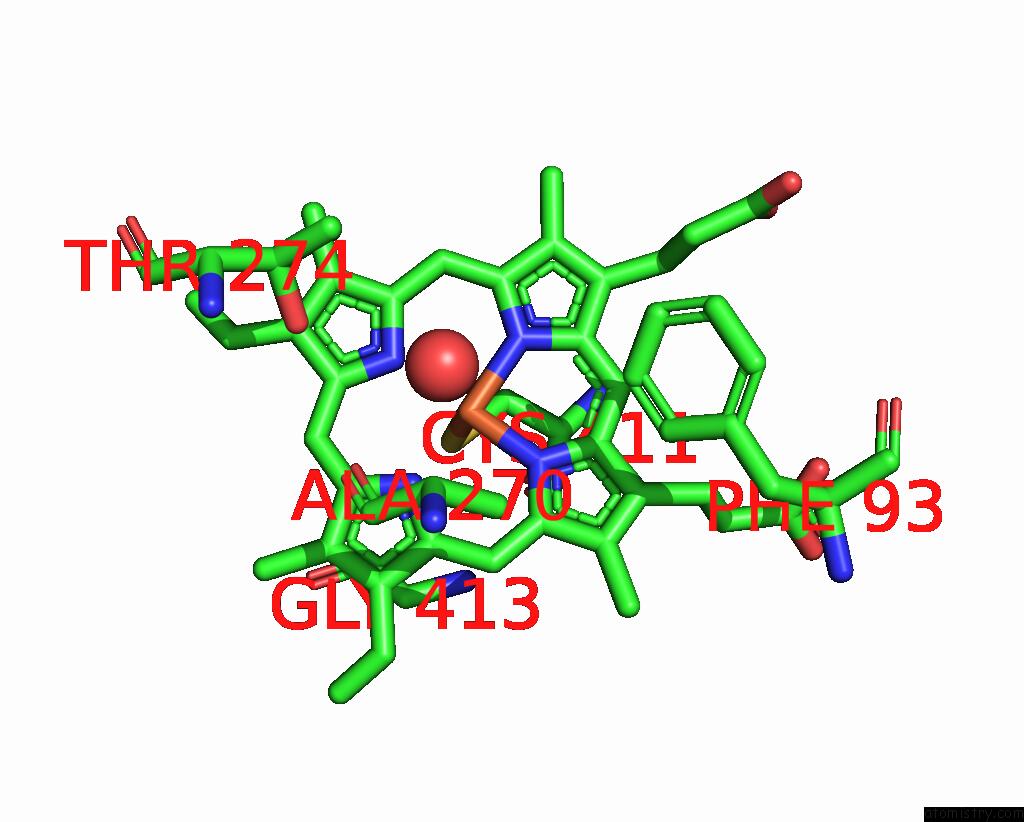
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid within 5.0Å range:
|
Iron binding site 4 out of 4 in 7p6l
Go back to
Iron binding site 4 out
of 4 in the Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid

Mono view
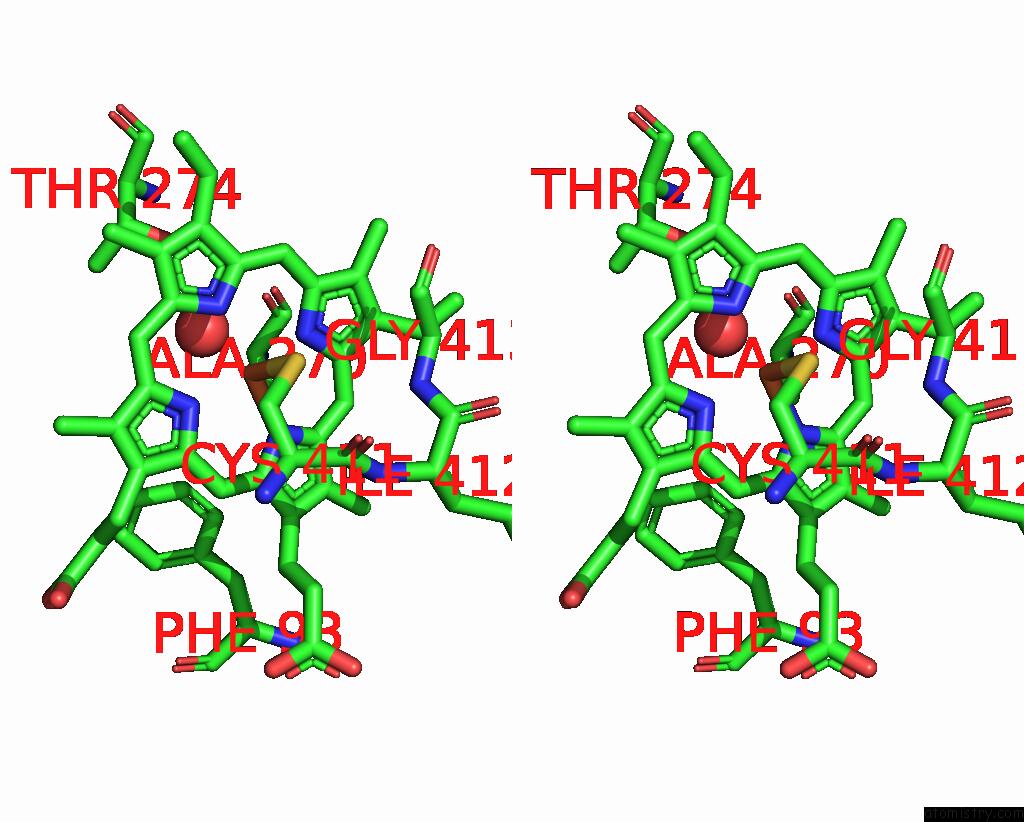
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Heme Domain of CYP505A30, A Fungal Hydroxylase From Myceliophthora Thermophila, Bound to Dodecanoic Acid within 5.0Å range:
|
Reference:
J.C.Aschenbrenner,
A.C.Ebrecht,
C.Tolmie,
M.S.Smit,
D.J.Opperman.
Structure of the Fungal Hydroxylase, CYP505A30, and Rational Transfer of Mutation Data From CYP102A1 to Alter Regioselectivity Catalysis Science and V. 11 7359 2021TECHNOLOGY.
ISSN: ESSN 2044-4761
DOI: 10.1039/D1CY01348C
Page generated: Thu Aug 7 02:01:13 2025
ISSN: ESSN 2044-4761
DOI: 10.1039/D1CY01348C
Last articles
Fe in 7R4FFe in 7R8T
Fe in 7R4Z
Fe in 7R8S
Fe in 7R4D
Fe in 7R4L
Fe in 7R4C
Fe in 7R48
Fe in 7R47
Fe in 7R46