Iron »
PDB 7p7l-7pr2 »
7pd2 »
Iron in PDB 7pd2: Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans
Protein crystallography data
The structure of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans, PDB code: 7pd2
was solved by
P.Amara,
C.Saragaglia,
J.-M.Mouesca,
L.Martin,
Y.Nicolet,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.46 / 1.99 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 175.09, 49.33, 85.21, 90, 96.91, 90 |
| R / Rfree (%) | 23 / 26.9 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans
(pdb code 7pd2). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 8 binding sites of Iron where determined in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans, PDB code: 7pd2:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Iron where determined in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans, PDB code: 7pd2:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Iron binding site 1 out of 8 in 7pd2
Go back to
Iron binding site 1 out
of 8 in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans
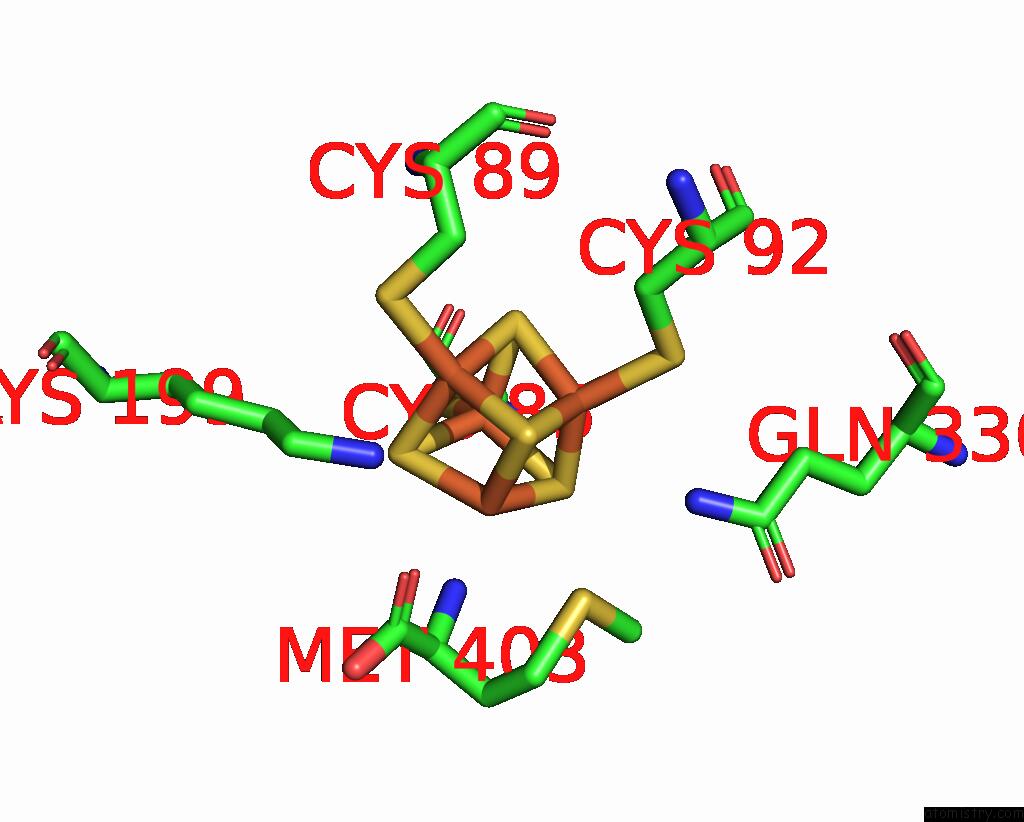
Mono view
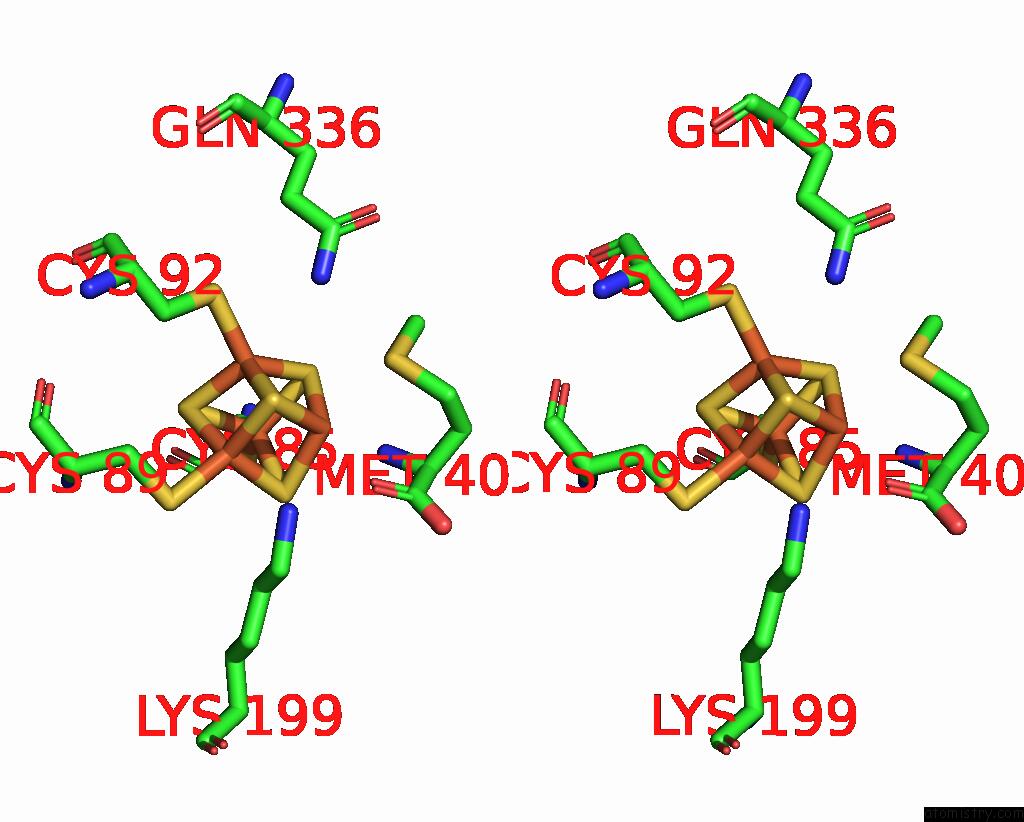
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans within 5.0Å range:
|
Iron binding site 2 out of 8 in 7pd2
Go back to
Iron binding site 2 out
of 8 in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans
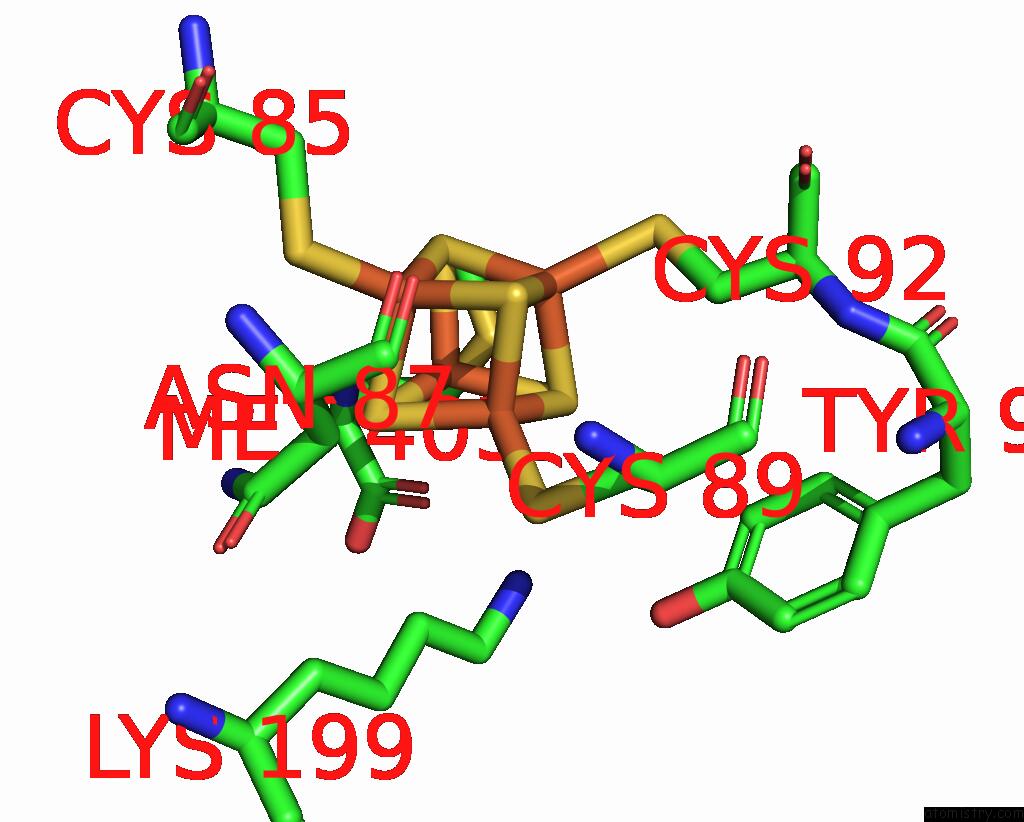
Mono view
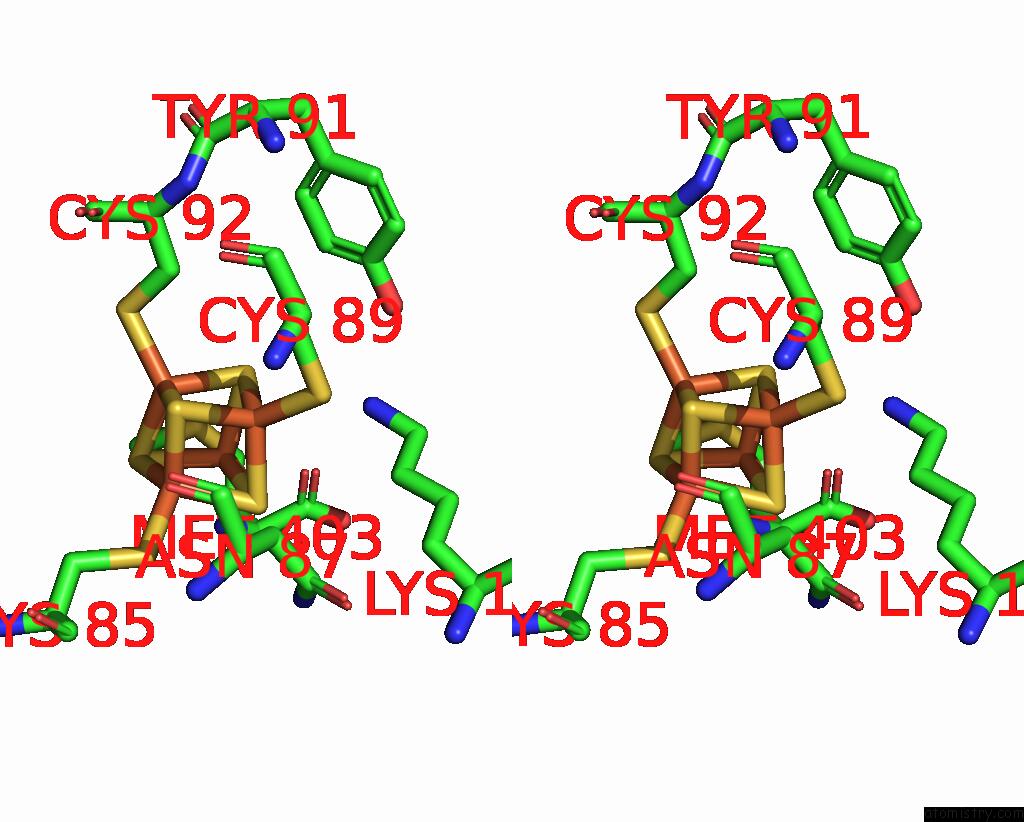
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans within 5.0Å range:
|
Iron binding site 3 out of 8 in 7pd2
Go back to
Iron binding site 3 out
of 8 in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans

Mono view
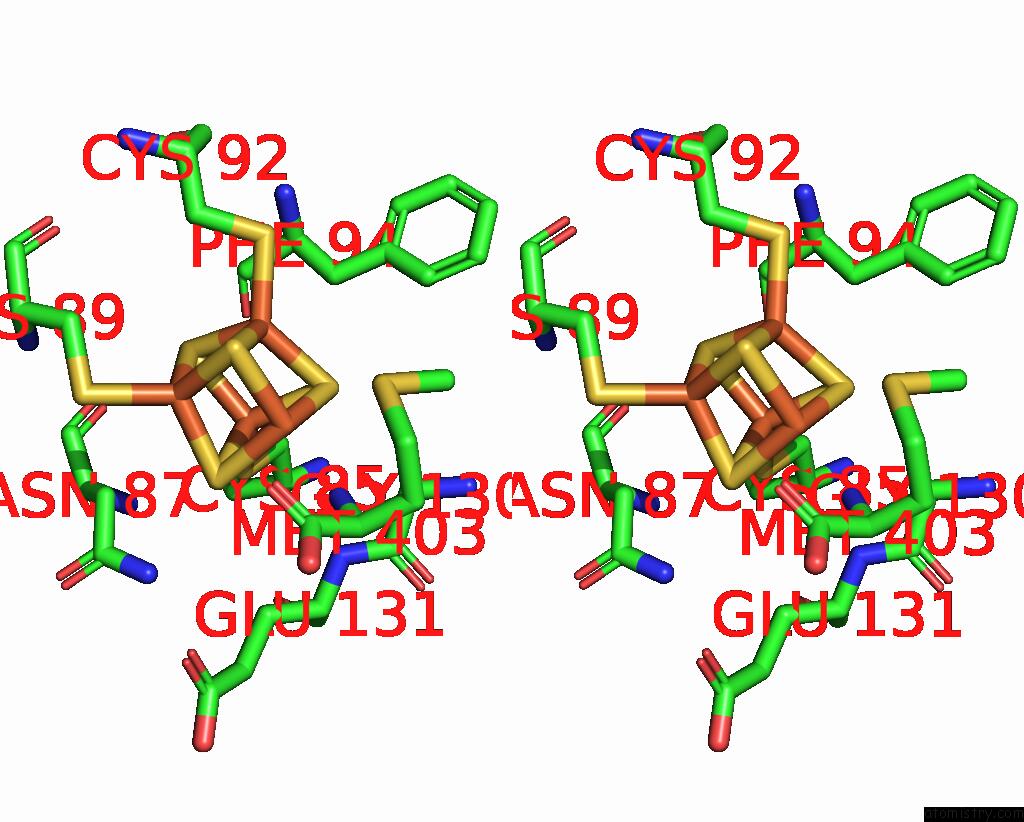
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans within 5.0Å range:
|
Iron binding site 4 out of 8 in 7pd2
Go back to
Iron binding site 4 out
of 8 in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans
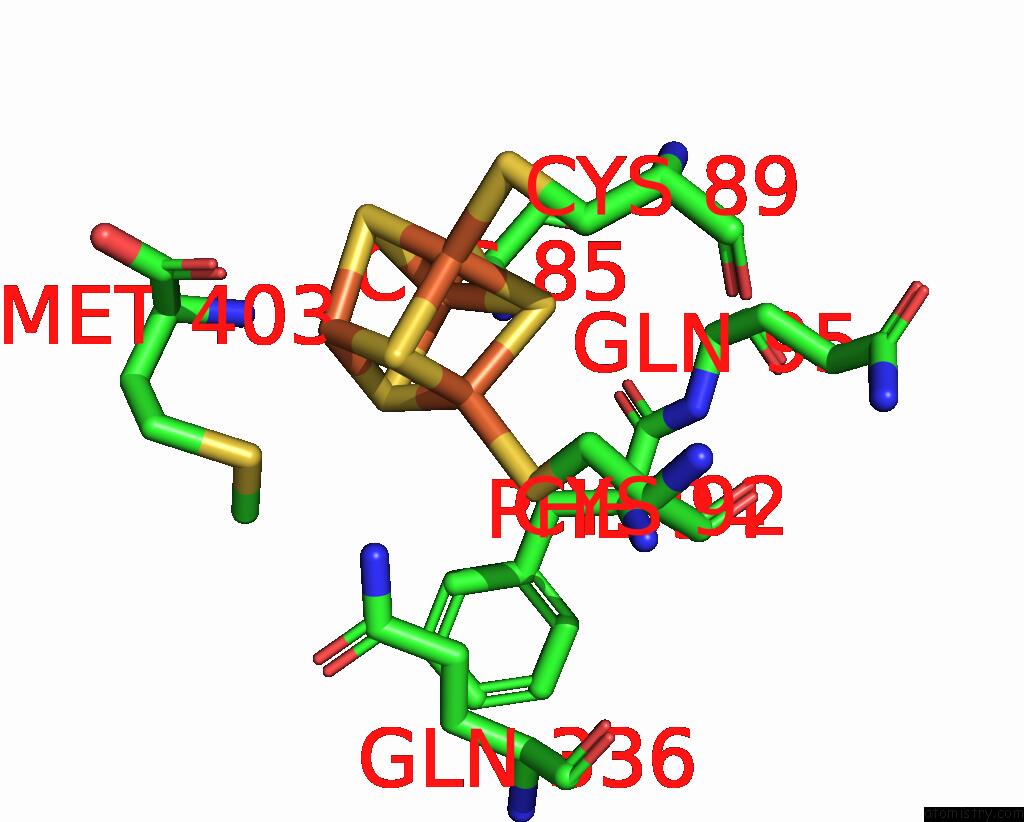
Mono view
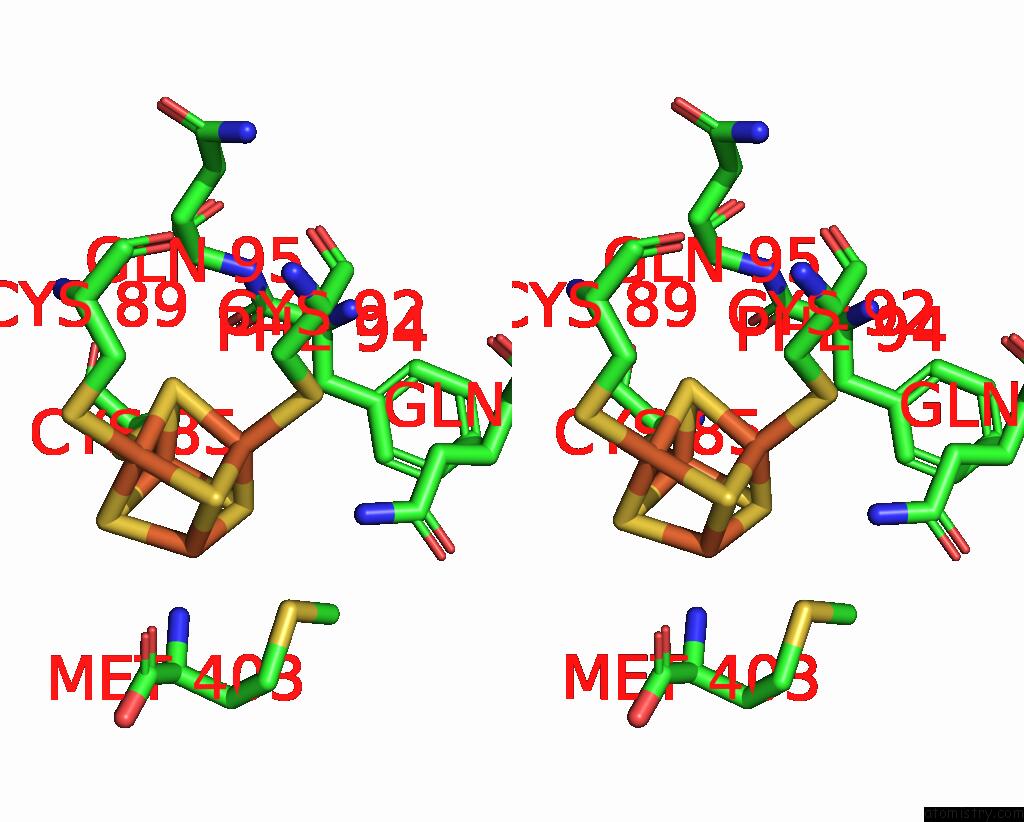
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans within 5.0Å range:
|
Iron binding site 5 out of 8 in 7pd2
Go back to
Iron binding site 5 out
of 8 in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans
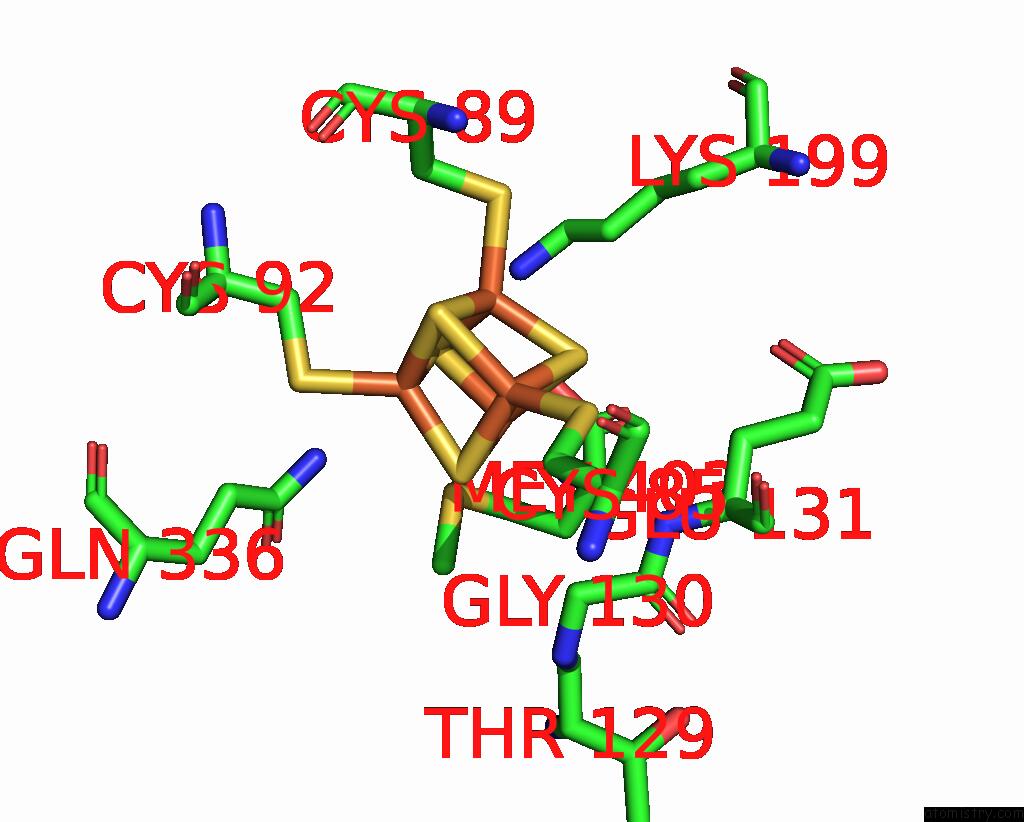
Mono view
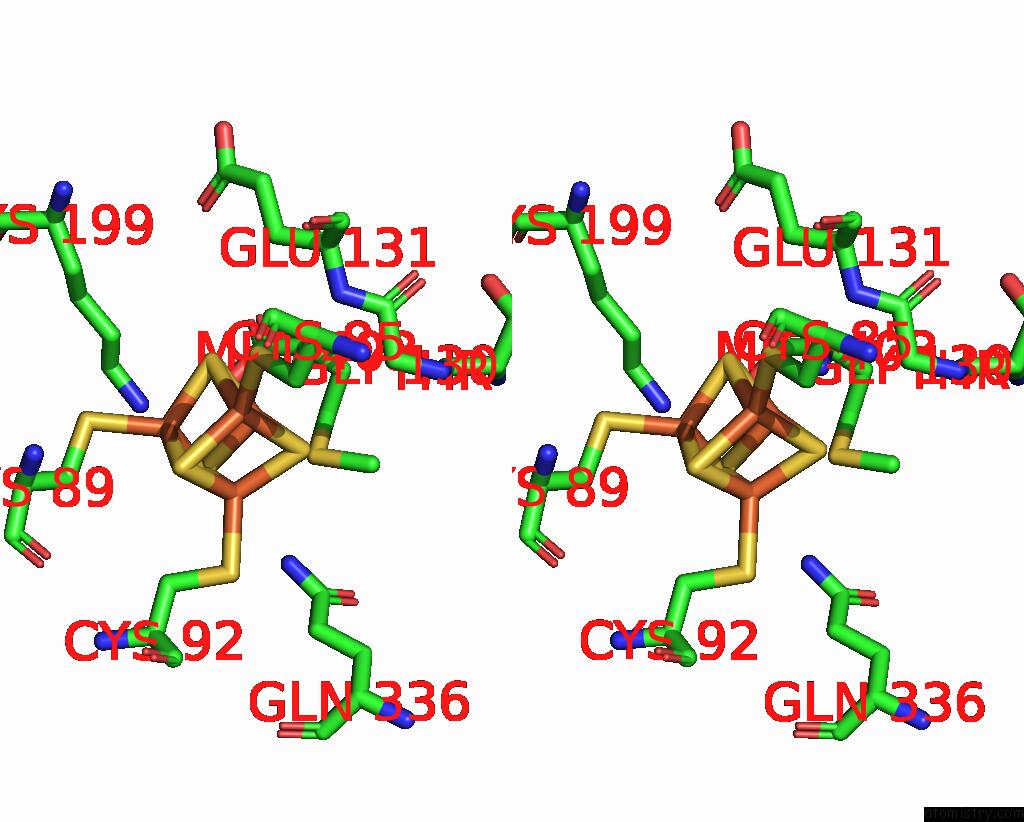
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans within 5.0Å range:
|
Iron binding site 6 out of 8 in 7pd2
Go back to
Iron binding site 6 out
of 8 in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans

Mono view
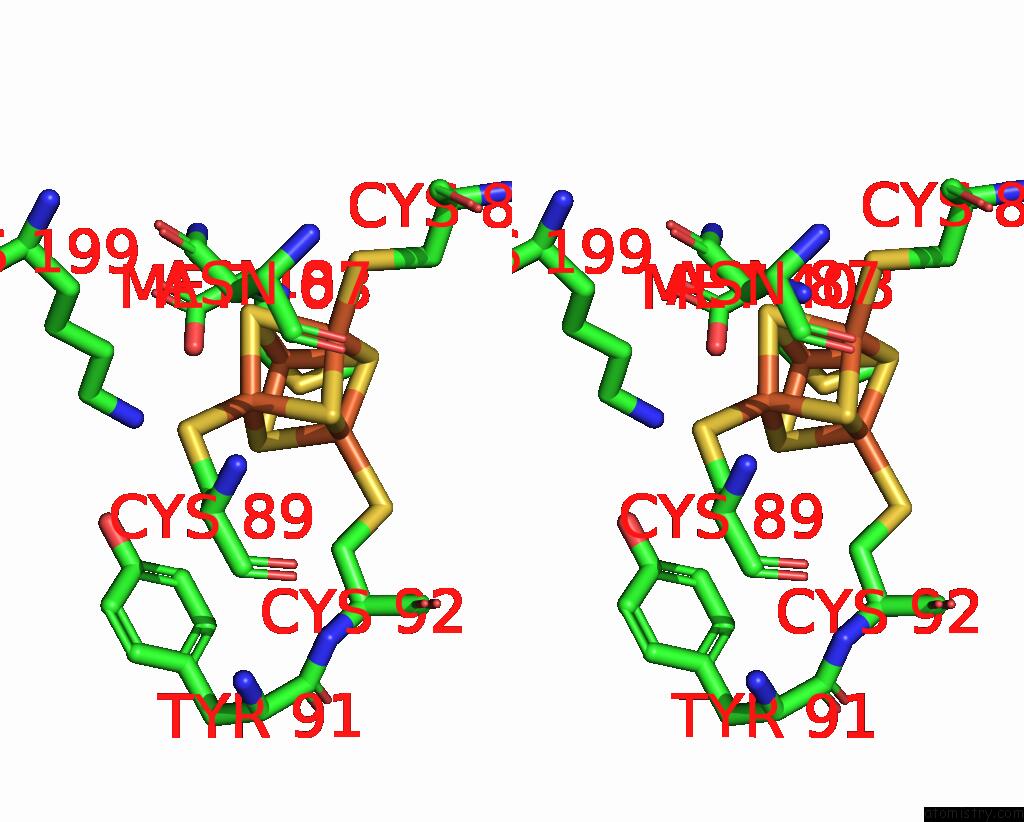
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans within 5.0Å range:
|
Iron binding site 7 out of 8 in 7pd2
Go back to
Iron binding site 7 out
of 8 in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans
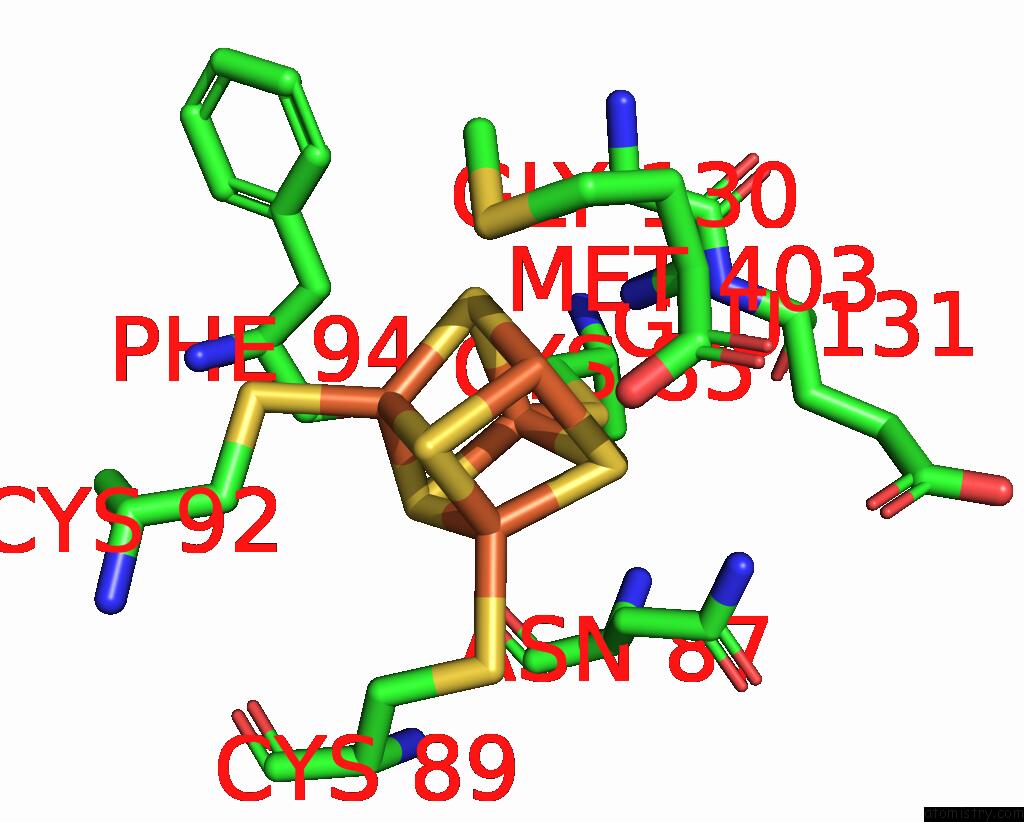
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans within 5.0Å range:
|
Iron binding site 8 out of 8 in 7pd2
Go back to
Iron binding site 8 out
of 8 in the Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans
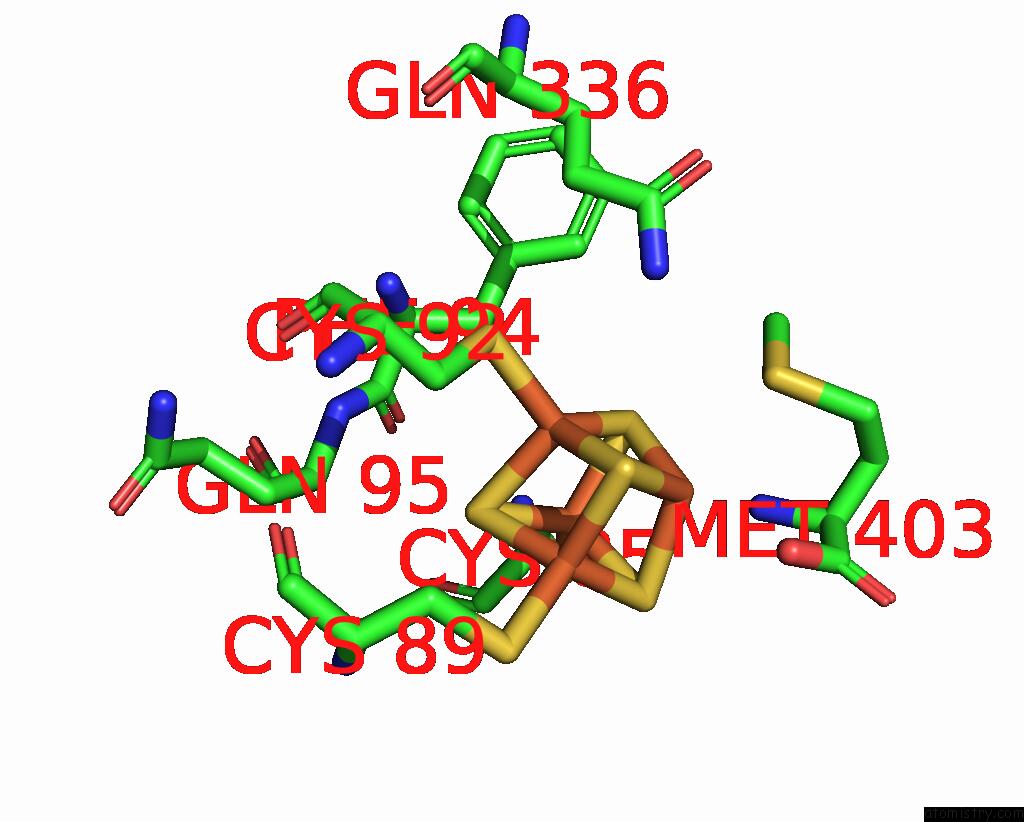
Mono view
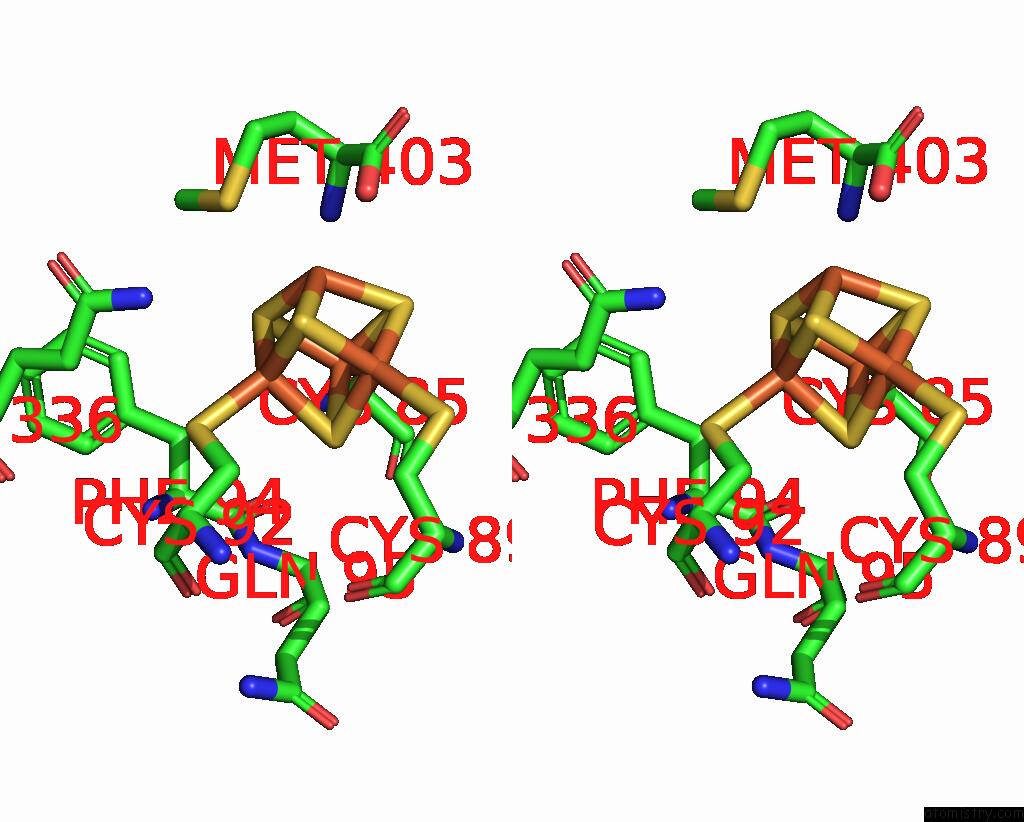
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Crystal Structure of the Substrate-Free Radical Sam Tyrosine Lyase Thih (2-Iminoacetate Synthase) From Thermosinus Carboxydivorans within 5.0Å range:
|
Reference:
P.Amara,
C.Saragaglia,
J.M.Mouesca,
L.Martin,
Y.Nicolet.
L-Tyrosine-Bound Thih Structure Reveals C-C Bond Break Differences Within Radical Sam Aromatic Amino Acid Lyases. Nat Commun V. 13 2284 2022.
ISSN: ESSN 2041-1723
PubMed: 35477710
DOI: 10.1038/S41467-022-29980-4
Page generated: Thu Aug 7 02:46:17 2025
ISSN: ESSN 2041-1723
PubMed: 35477710
DOI: 10.1038/S41467-022-29980-4
Last articles
Mg in 1VQNMg in 1W25
Mg in 1W23
Mg in 1W1Z
Mg in 1W1W
Mg in 1W0N
Mg in 1W0K
Mg in 1W0J
Mg in 1W0H
Mg in 1VZM