Iron »
PDB 7pr3-7qhm »
7q4f »
Iron in PDB 7q4f: Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme
Protein crystallography data
The structure of Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme, PDB code: 7q4f
was solved by
H.Michlits,
N.Valente,
G.Mlynek,
S.Hofbauer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 43.51 / 2.15 |
| Space group | P 3 1 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 141.809, 141.809, 124.863, 90, 90, 120 |
| R / Rfree (%) | 14 / 18.2 |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme
(pdb code 7q4f). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 5 binding sites of Iron where determined in the Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme, PDB code: 7q4f:
Jump to Iron binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Iron where determined in the Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme, PDB code: 7q4f:
Jump to Iron binding site number: 1; 2; 3; 4; 5;
Iron binding site 1 out of 5 in 7q4f
Go back to
Iron binding site 1 out
of 5 in the Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme within 5.0Å range:
|
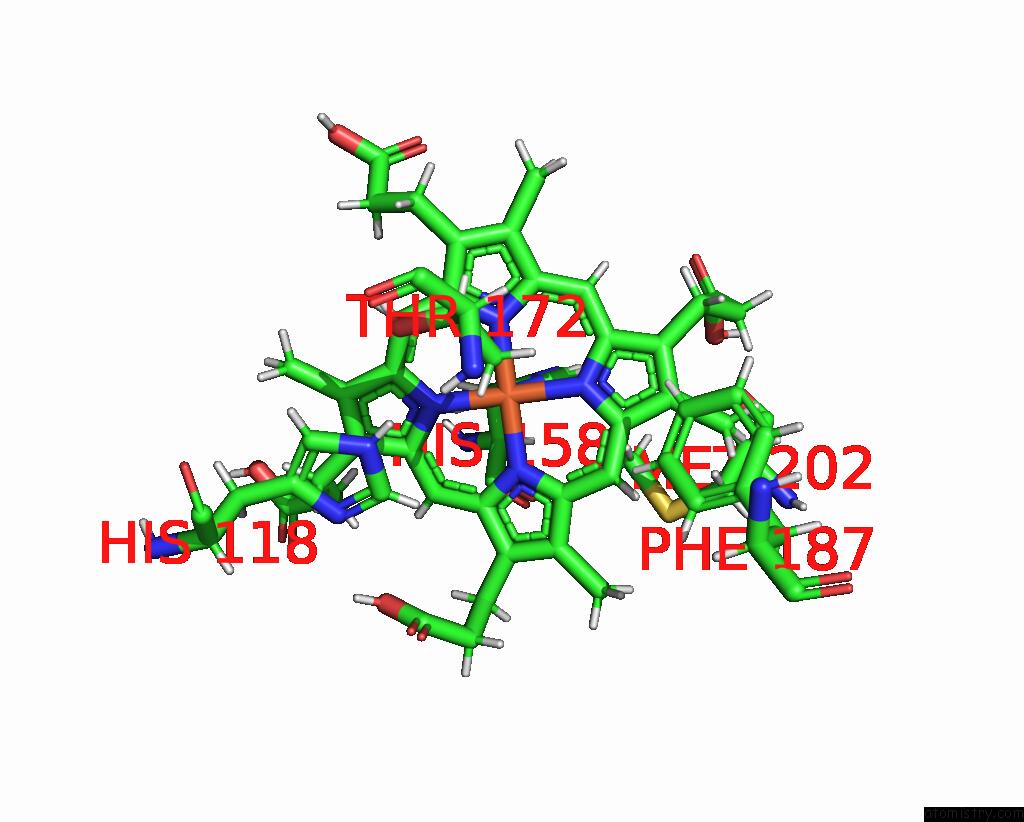
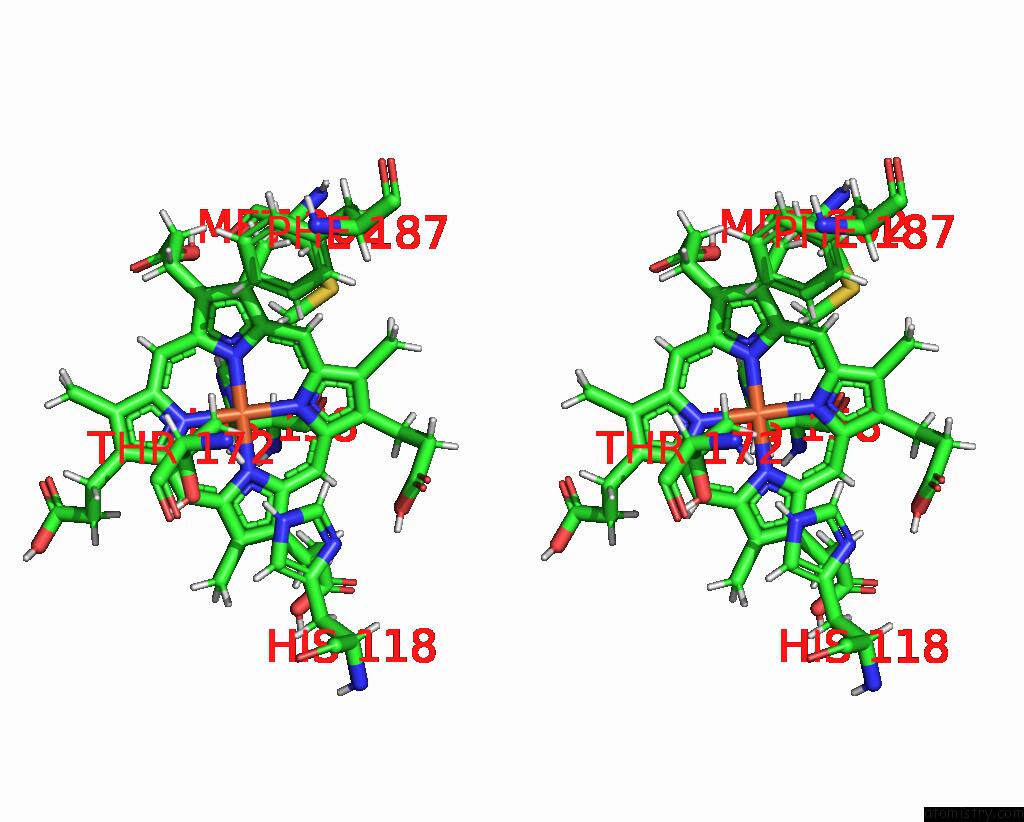
Iron binding site 2 out of 5 in 7q4f
Go back to
Iron binding site 2 out
of 5 in the Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme
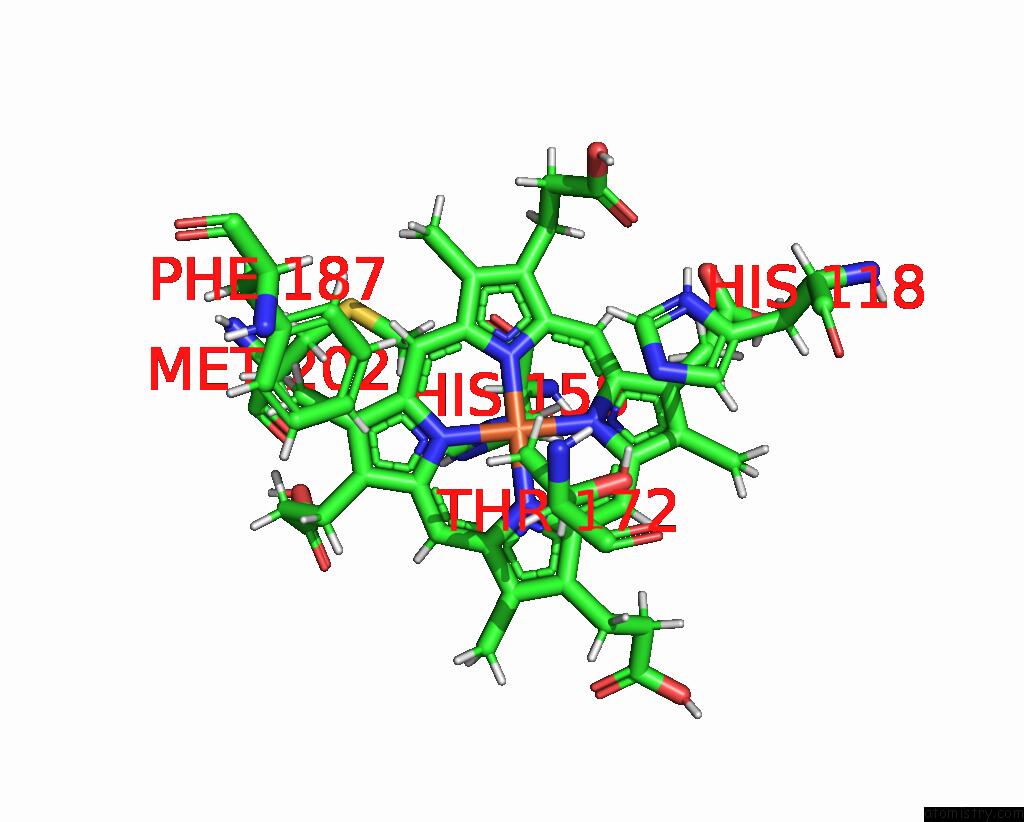
Mono view
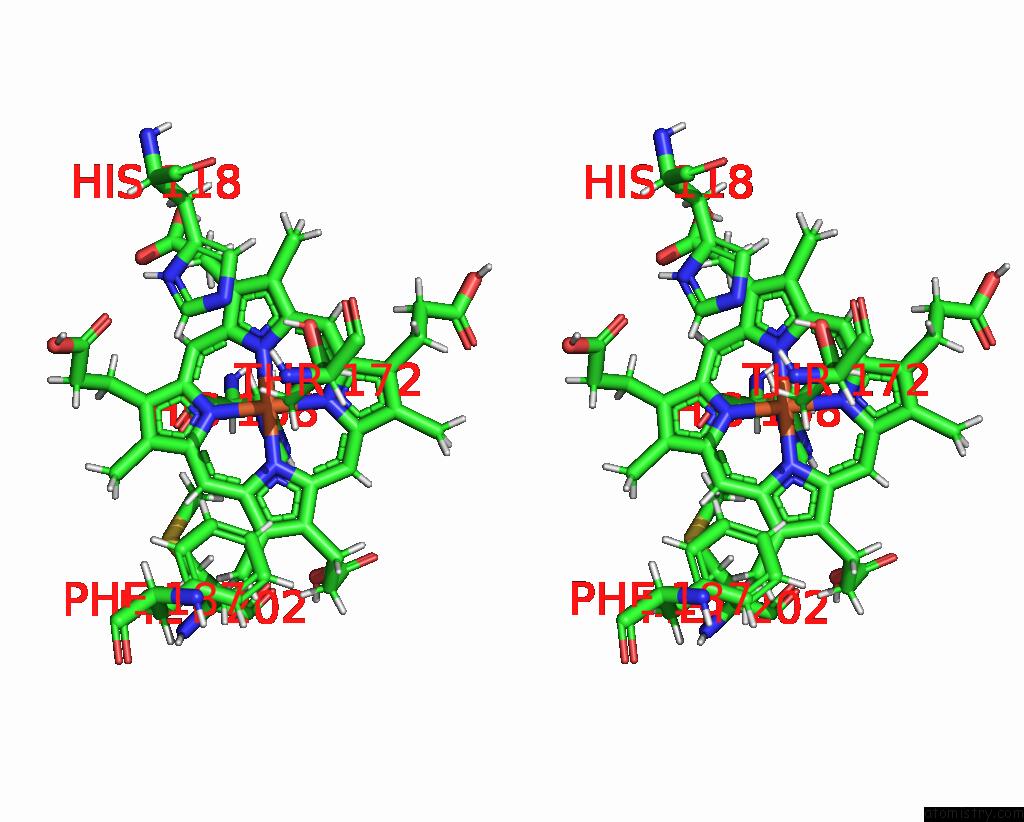
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme within 5.0Å range:
|
Iron binding site 3 out of 5 in 7q4f
Go back to
Iron binding site 3 out
of 5 in the Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme within 5.0Å range:
|
Iron binding site 4 out of 5 in 7q4f
Go back to
Iron binding site 4 out
of 5 in the Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme
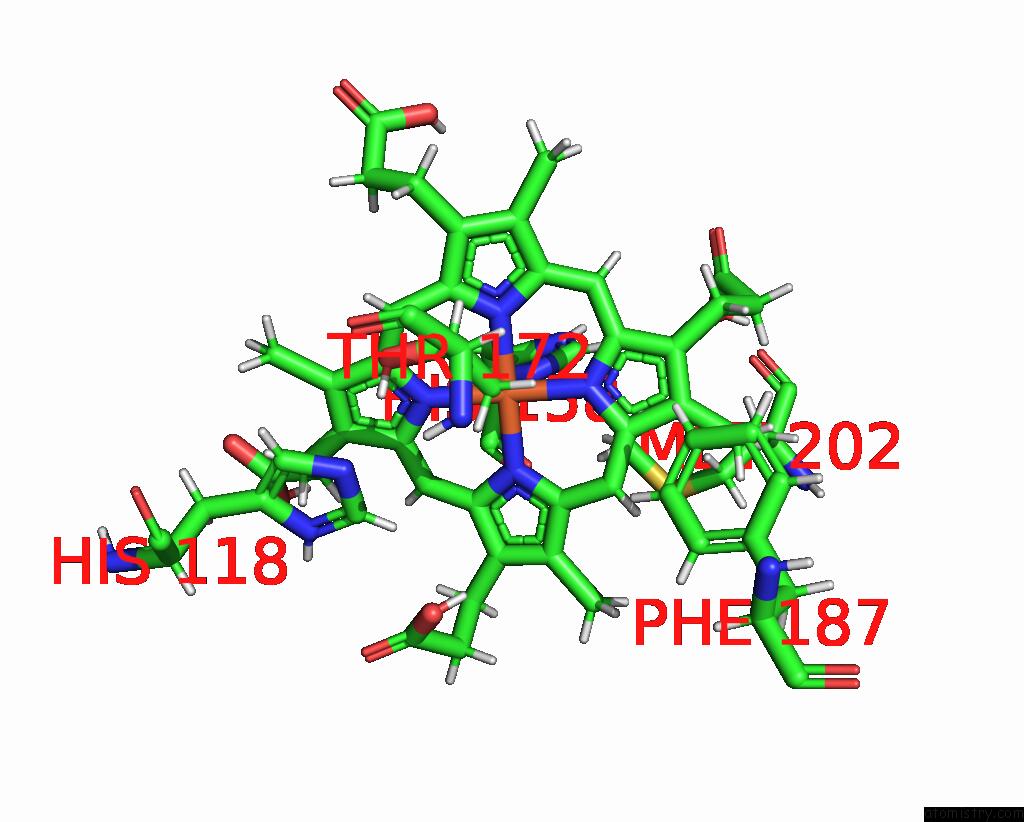
Mono view
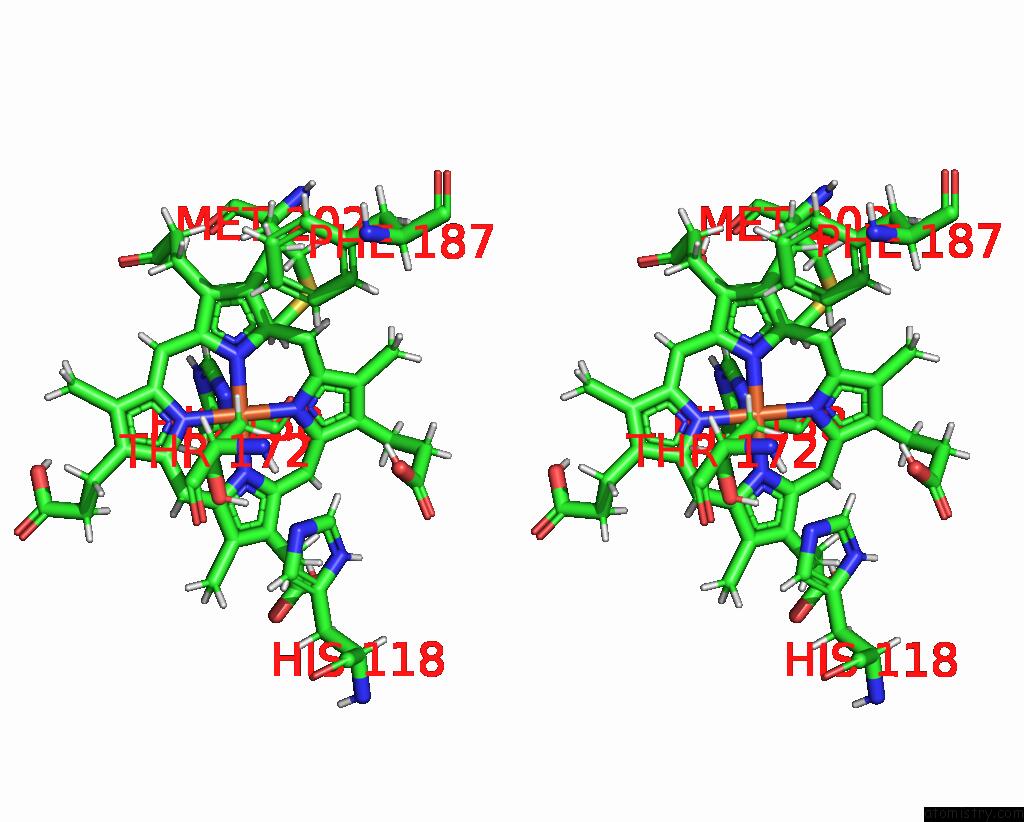
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme within 5.0Å range:
|
Iron binding site 5 out of 5 in 7q4f
Go back to
Iron binding site 5 out
of 5 in the Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme
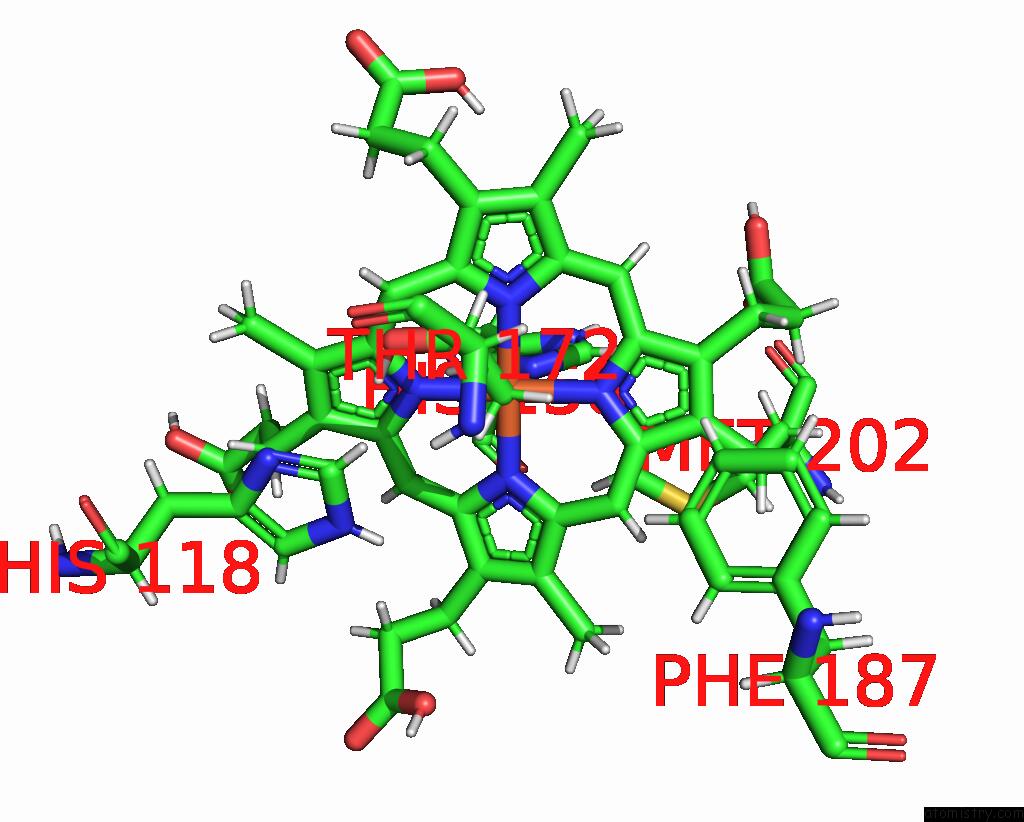
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Structure of Coproheme Decarboxylase From Corynebacterium Dipththeriae W183Y Mutant in Complex with Coproheme within 5.0Å range:
|
Reference:
H.Michlits,
N.Valente,
G.Mlynek,
S.Hofbauer.
Initial Steps to Engineer Coproheme Decarboxylase to Obtain Stereospecific Monovinyl, Monopropionyl Deuterohemes. Front Bioeng Biotechnol V. 9 07678 2021.
ISSN: ISSN 2296-4185
PubMed: 35141216
DOI: 10.3389/FBIOE.2021.807678
Page generated: Thu Aug 7 03:03:49 2025
ISSN: ISSN 2296-4185
PubMed: 35141216
DOI: 10.3389/FBIOE.2021.807678
Last articles
K in 8WUWK in 8WUX
K in 8X0S
K in 8X4R
K in 8WGR
K in 8W9V
K in 8W9O
K in 8WA5
K in 8VJT
K in 8W9D