Iron »
PDB 7qho-7r2s »
7qr6 »
Iron in PDB 7qr6: Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant
Protein crystallography data
The structure of Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant, PDB code: 7qr6
was solved by
L.Alvigini,
A.Mattevi,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.04 / 2.90 |
| Space group | C 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 178.471, 187.984, 105.867, 90, 90, 90 |
| R / Rfree (%) | 21.4 / 29.1 |
Iron Binding Sites:
The binding sites of Iron atom in the Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant
(pdb code 7qr6). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 3 binding sites of Iron where determined in the Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant, PDB code: 7qr6:
Jump to Iron binding site number: 1; 2; 3;
In total 3 binding sites of Iron where determined in the Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant, PDB code: 7qr6:
Jump to Iron binding site number: 1; 2; 3;
Iron binding site 1 out of 3 in 7qr6
Go back to
Iron binding site 1 out
of 3 in the Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant
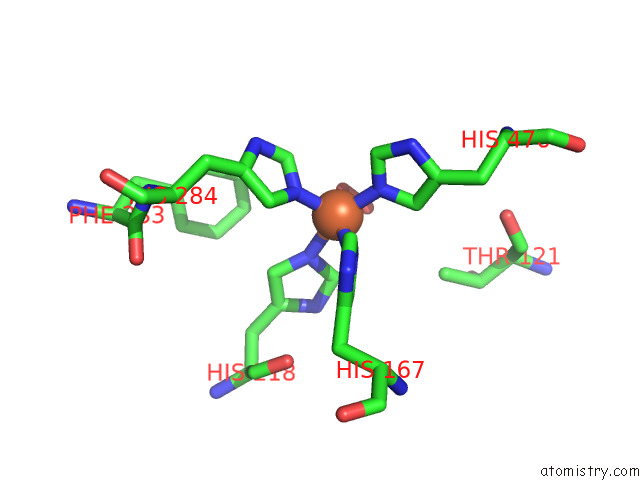
Mono view
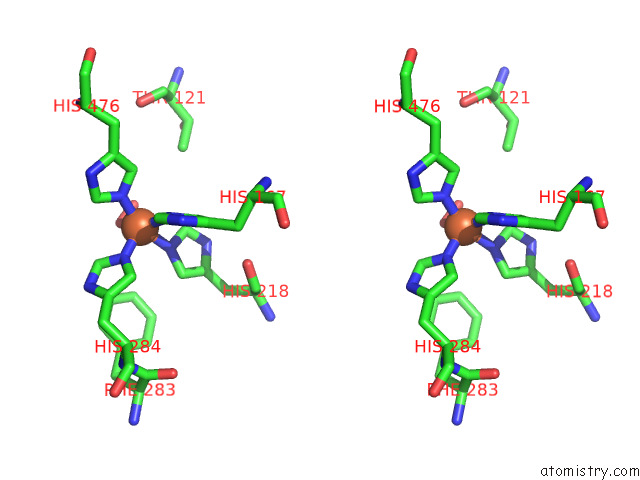
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant within 5.0Å range:
|
Iron binding site 2 out of 3 in 7qr6
Go back to
Iron binding site 2 out
of 3 in the Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant
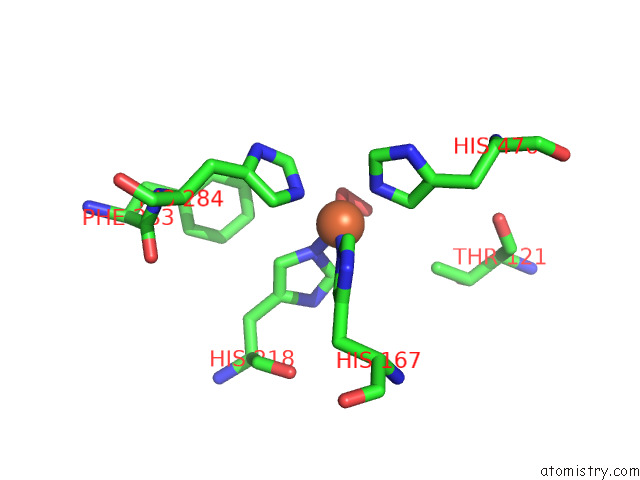
Mono view
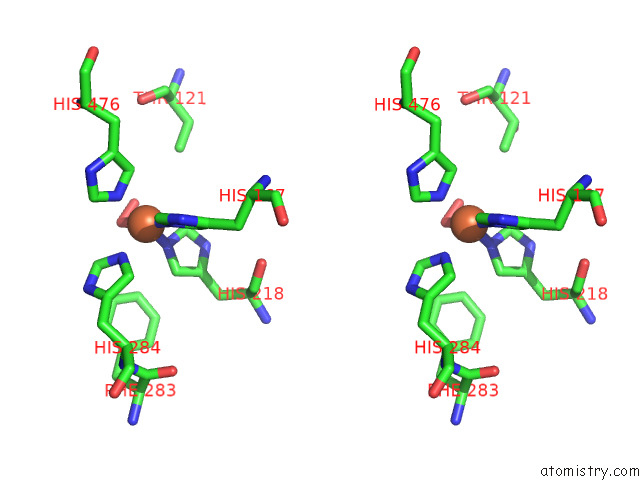
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant within 5.0Å range:
|
Iron binding site 3 out of 3 in 7qr6
Go back to
Iron binding site 3 out
of 3 in the Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant
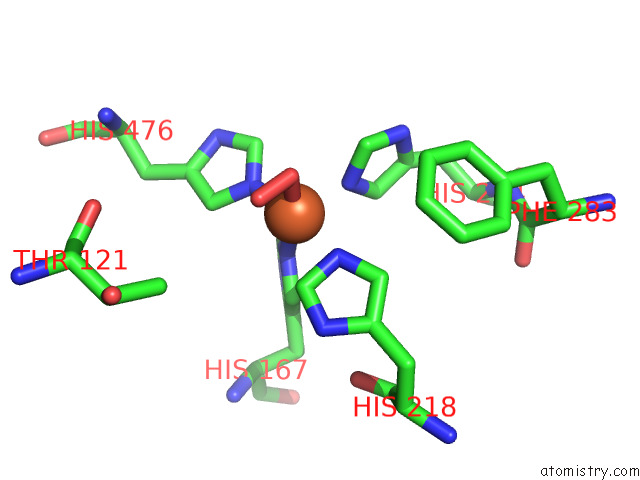
Mono view
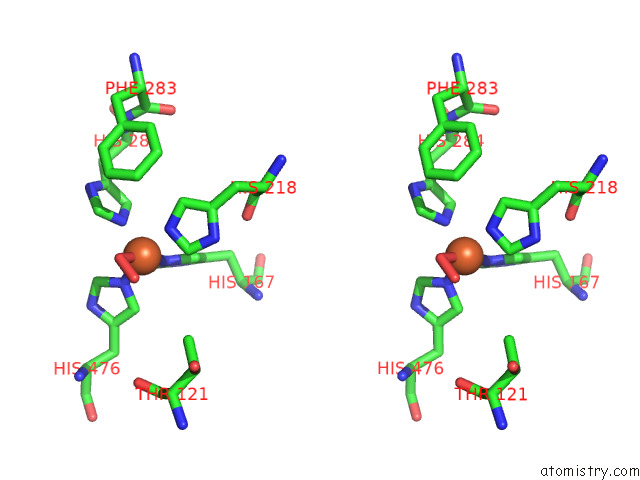
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Stilbene Dioxygenase (NOV1) From Novosphingobium Aromaticivorans: SER283PHE Mutant within 5.0Å range:
|
Reference:
M.De Simone,
L.Alvigini,
L.Alonso-Cotchico,
V.Brissos,
J.Caroli,
M.F.Lucas,
E.Monza,
E.P.Melo,
A.Mattevi,
L.O.Martins.
Rationally Guided Improvement of NOV1 Dioxygenase For the Conversion of Lignin-Derived Isoeugenol to Vanillin. Biochemistry 2022.
ISSN: ISSN 0006-2960
PubMed: 35687874
DOI: 10.1021/ACS.BIOCHEM.2C00168
Page generated: Thu Aug 7 03:48:07 2025
ISSN: ISSN 0006-2960
PubMed: 35687874
DOI: 10.1021/ACS.BIOCHEM.2C00168
Last articles
Mg in 1K8RMg in 1K77
Mg in 1K72
Mg in 1K6N
Mg in 1K6L
Mg in 1K6D
Mg in 1K6E
Mg in 1K63
Mg in 1K68
Mg in 1K5G