Iron »
PDB 7sh5-7tc8 »
7sph »
Iron in PDB 7sph: Crystal Structure of Sperm Whale Myoglobin Variant SMB13(Pcaaf) in Space Group P21
Protein crystallography data
The structure of Crystal Structure of Sperm Whale Myoglobin Variant SMB13(Pcaaf) in Space Group P21, PDB code: 7sph
was solved by
J.P.Bacik,
R.Fasan,
N.Ando,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 31.15 / 1.30 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 49.044, 41.628, 50.887, 90, 112.7, 90 |
| R / Rfree (%) | 13.4 / 16 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of Sperm Whale Myoglobin Variant SMB13(Pcaaf) in Space Group P21
(pdb code 7sph). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Crystal Structure of Sperm Whale Myoglobin Variant SMB13(Pcaaf) in Space Group P21, PDB code: 7sph:
In total only one binding site of Iron was determined in the Crystal Structure of Sperm Whale Myoglobin Variant SMB13(Pcaaf) in Space Group P21, PDB code: 7sph:
Iron binding site 1 out of 1 in 7sph
Go back to
Iron binding site 1 out
of 1 in the Crystal Structure of Sperm Whale Myoglobin Variant SMB13(Pcaaf) in Space Group P21
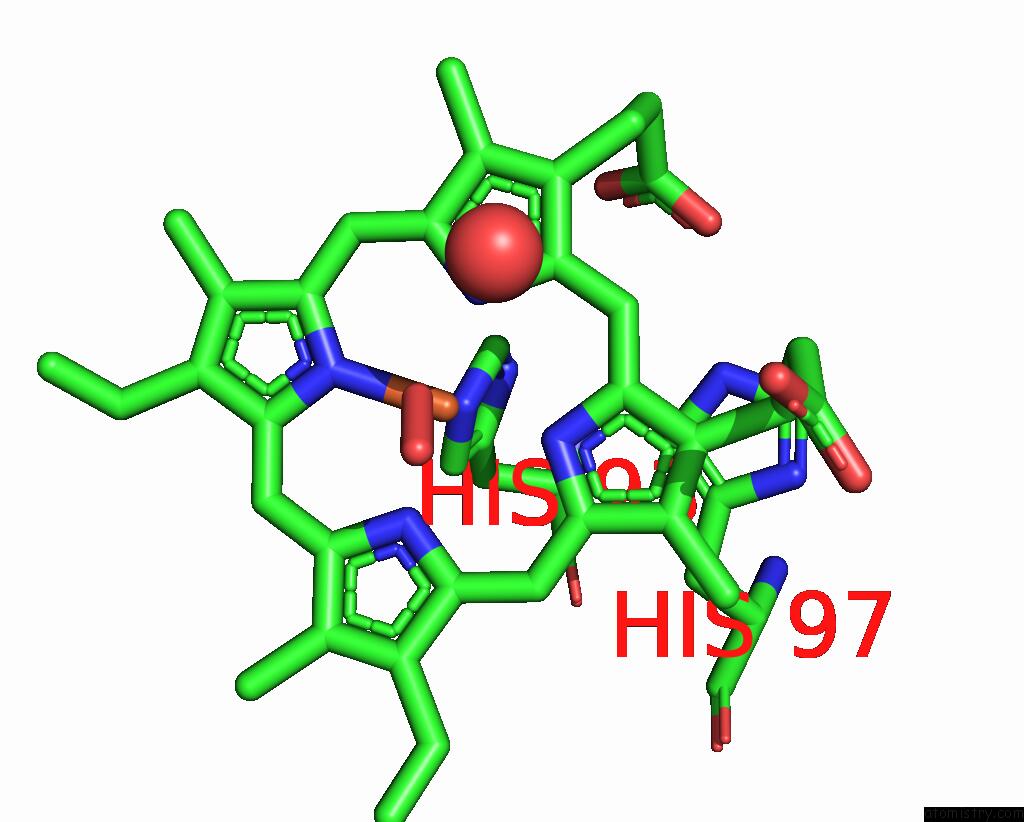
Mono view
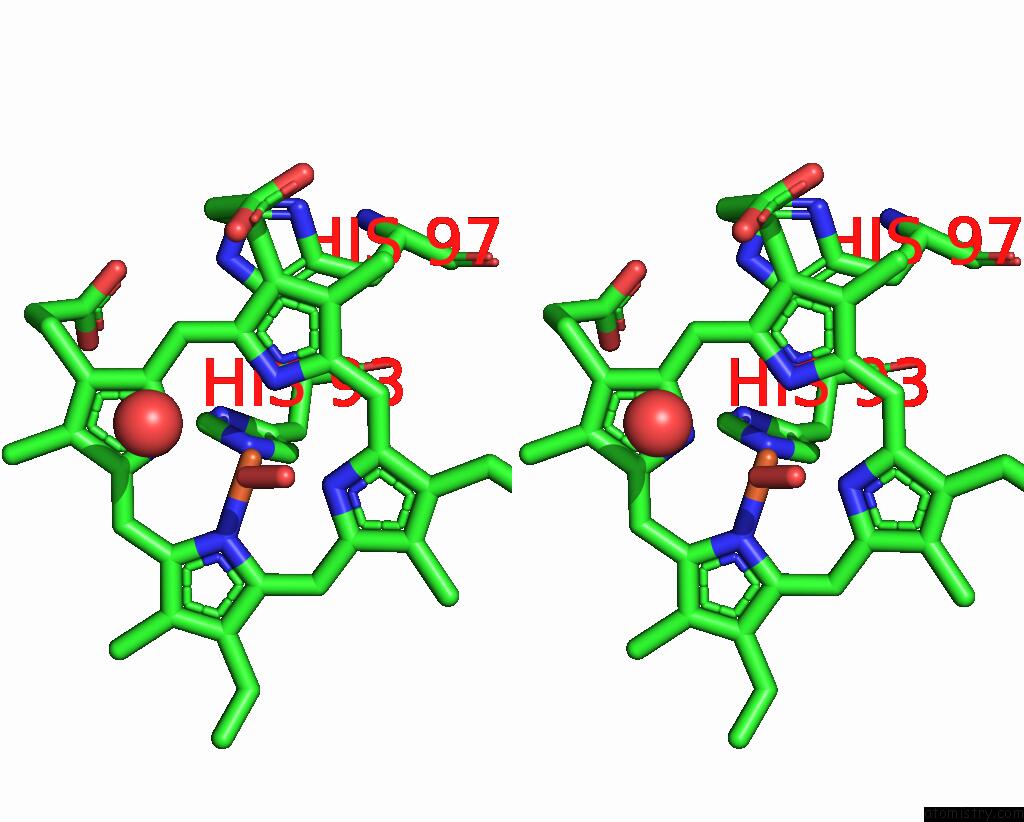
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of Sperm Whale Myoglobin Variant SMB13(Pcaaf) in Space Group P21 within 5.0Å range:
|
Reference:
J.A.Iannuzzelli,
J.P.Bacik,
E.J.Moore,
Z.Shen,
E.M.Irving,
D.A.Vargas,
S.D.Khare,
N.Ando,
R.Fasan.
Tuning Enzyme Thermostability Via Computationally Guided Covalent Stapling and Structural Basis of Enhanced Stabilization. Biochemistry V. 61 1041 2022.
ISSN: ISSN 0006-2960
PubMed: 35612958
DOI: 10.1021/ACS.BIOCHEM.2C00033
Page generated: Thu Aug 7 05:36:03 2025
ISSN: ISSN 0006-2960
PubMed: 35612958
DOI: 10.1021/ACS.BIOCHEM.2C00033
Last articles
Fe in 7VT2Fe in 7VXU
Fe in 7VY3
Fe in 7VXP
Fe in 7VXQ
Fe in 7VWJ
Fe in 7VW6
Fe in 7VX0
Fe in 7VVS
Fe in 7VW4