Iron »
PDB 7vqg-7w1t »
7vw6 »
Iron in PDB 7vw6: Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
Enzymatic activity of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
All present enzymatic activity of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1:
1.17.1.9; 1.2.1.2;
1.17.1.9; 1.2.1.2;
Other elements in 7vw6:
The structure of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 also contains other interesting chemical elements:
| Tungsten | (W) | 1 atom |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20;Binding sites:
The binding sites of Iron atom in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 (pdb code 7vw6). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 20 binding sites of Iron where determined in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1, PDB code: 7vw6:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 20 in 7vw6
Go back to
Iron binding site 1 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
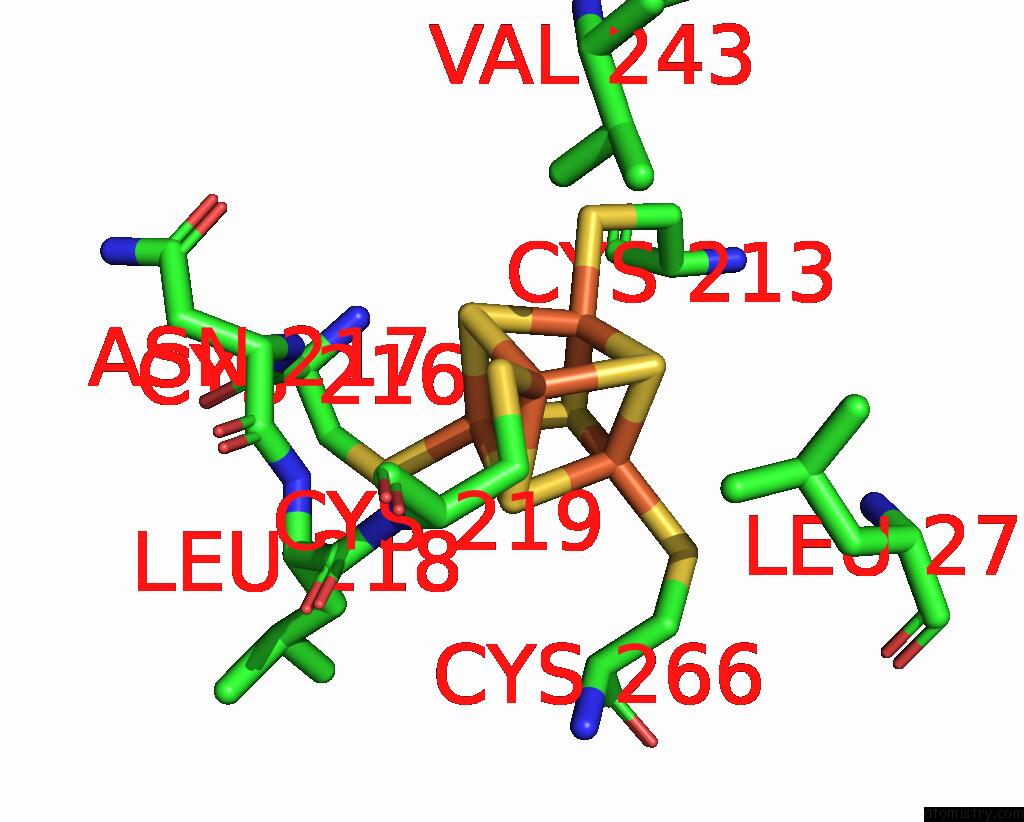
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
Iron binding site 2 out of 20 in 7vw6
Go back to
Iron binding site 2 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
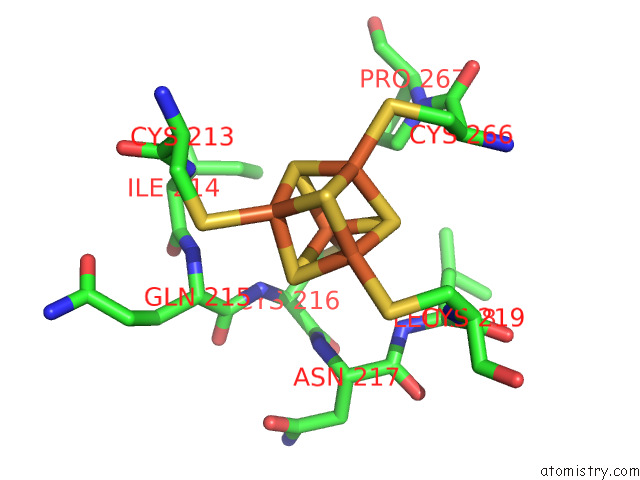
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
Iron binding site 3 out of 20 in 7vw6
Go back to
Iron binding site 3 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1

Mono view
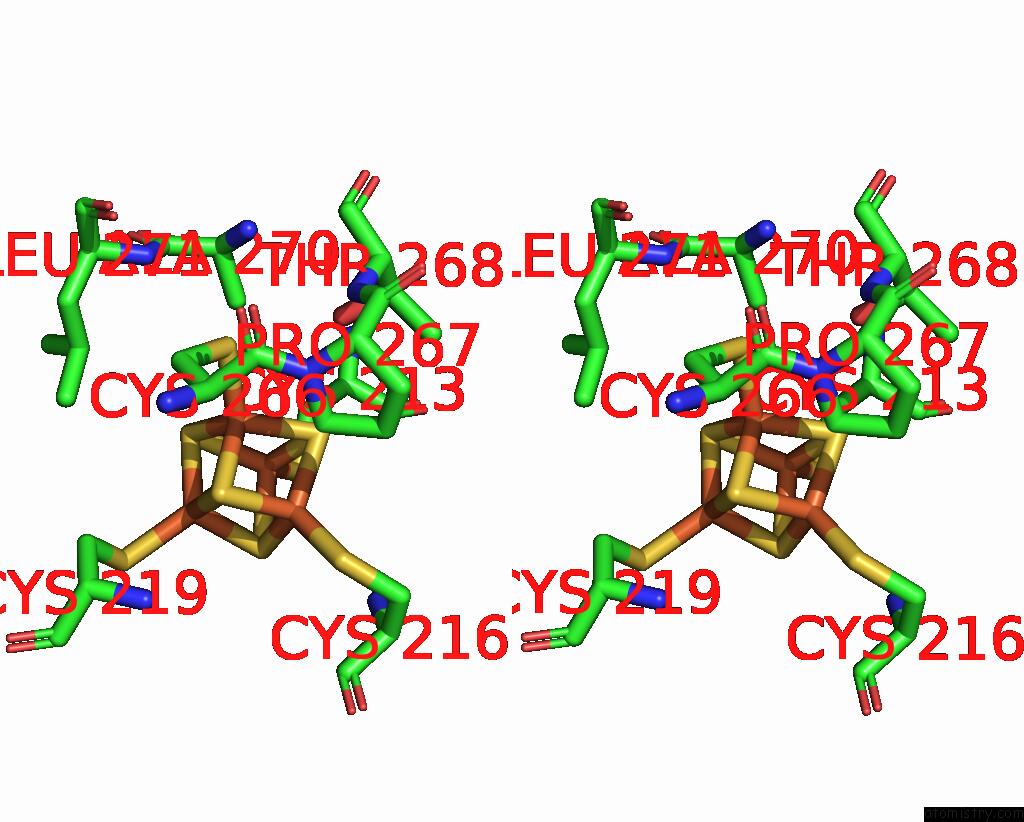
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
Iron binding site 4 out of 20 in 7vw6
Go back to
Iron binding site 4 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
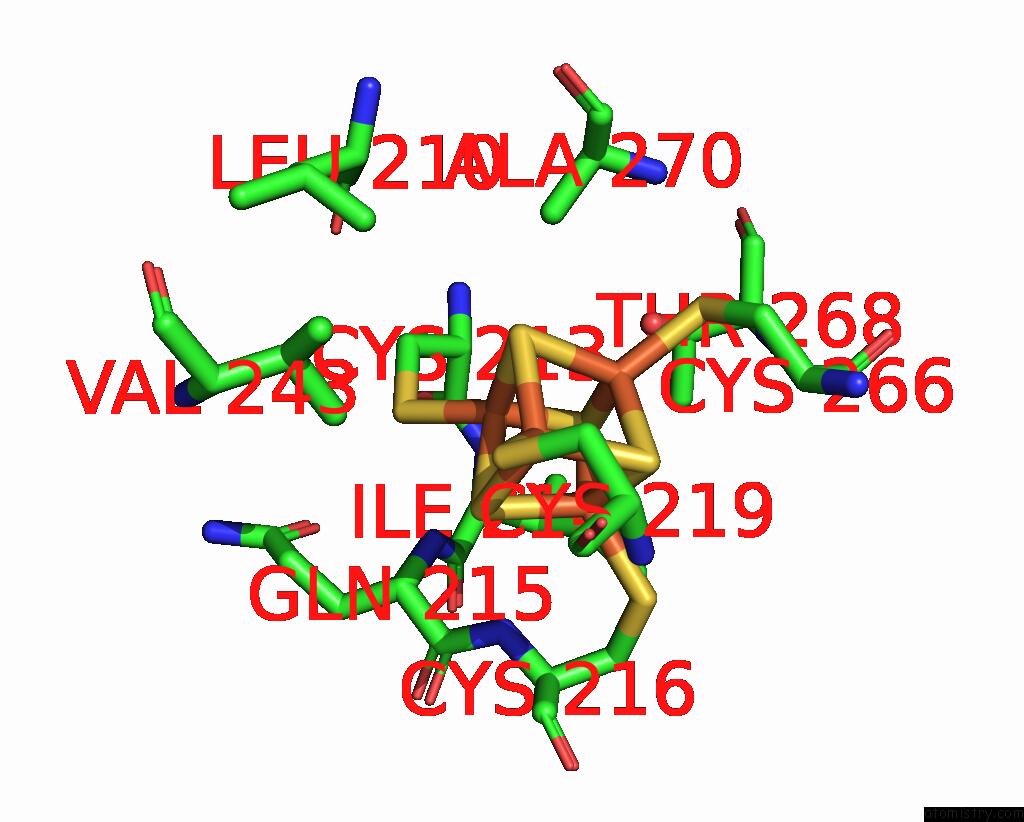
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
Iron binding site 5 out of 20 in 7vw6
Go back to
Iron binding site 5 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
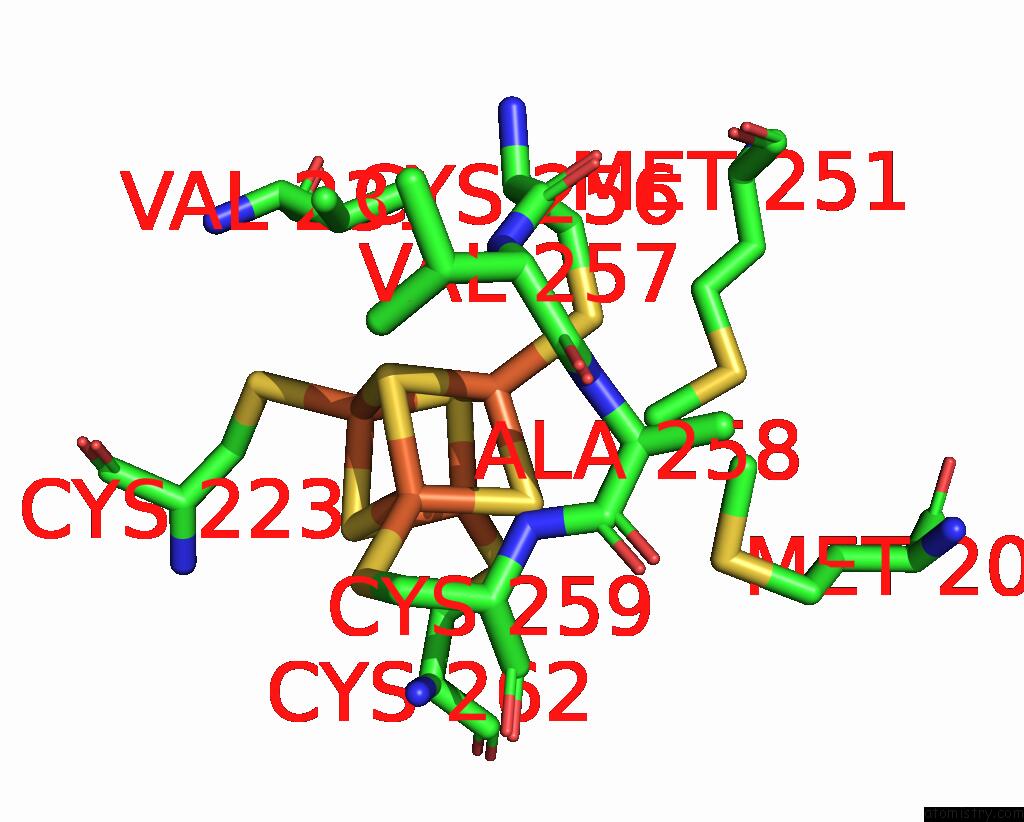
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
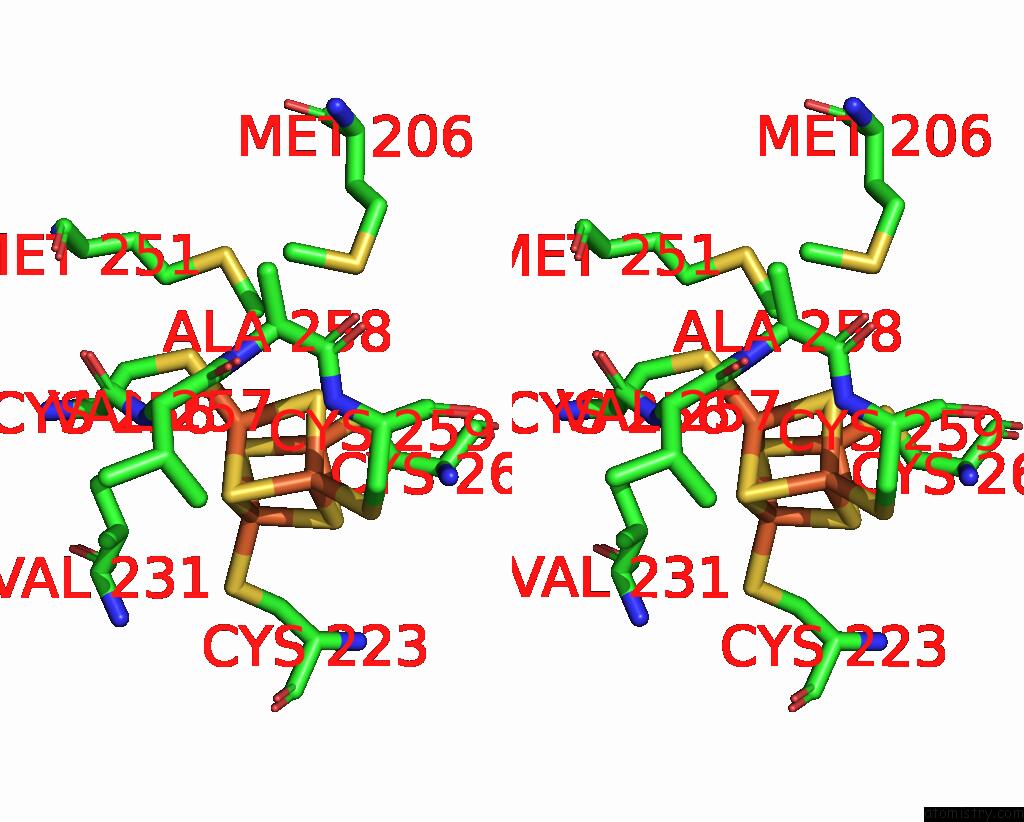
Iron binding site 6 out of 20 in 7vw6
Go back to
Iron binding site 6 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
Iron binding site 7 out of 20 in 7vw6
Go back to
Iron binding site 7 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
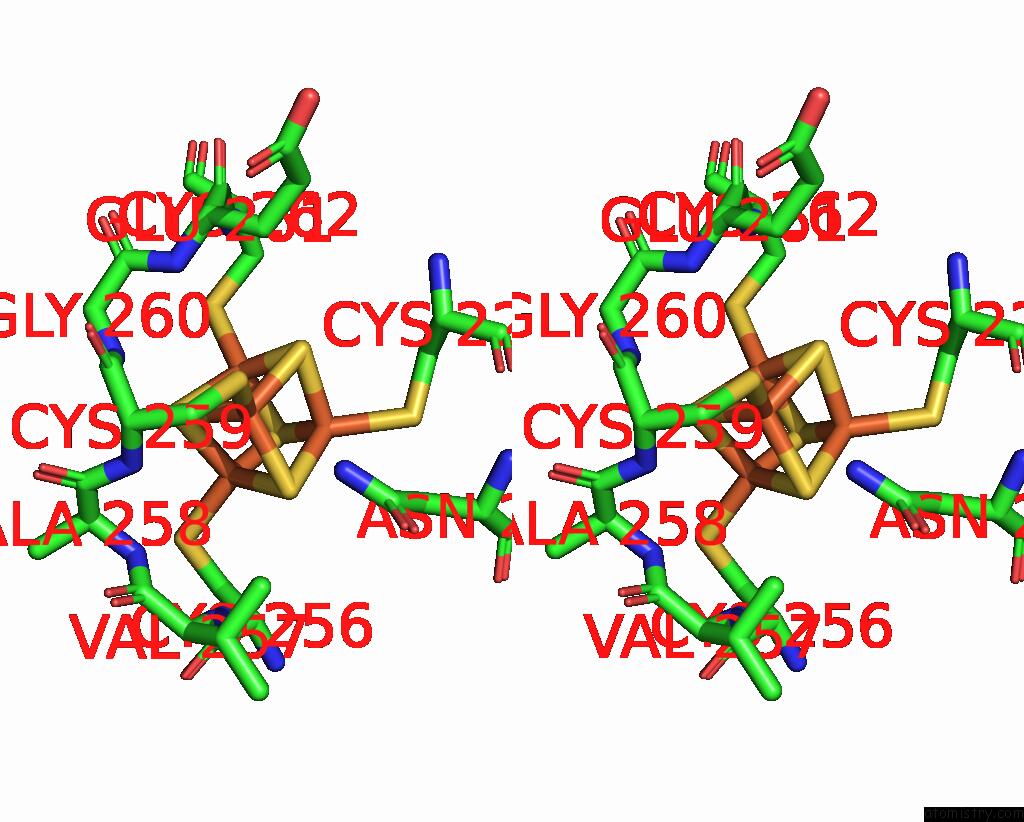
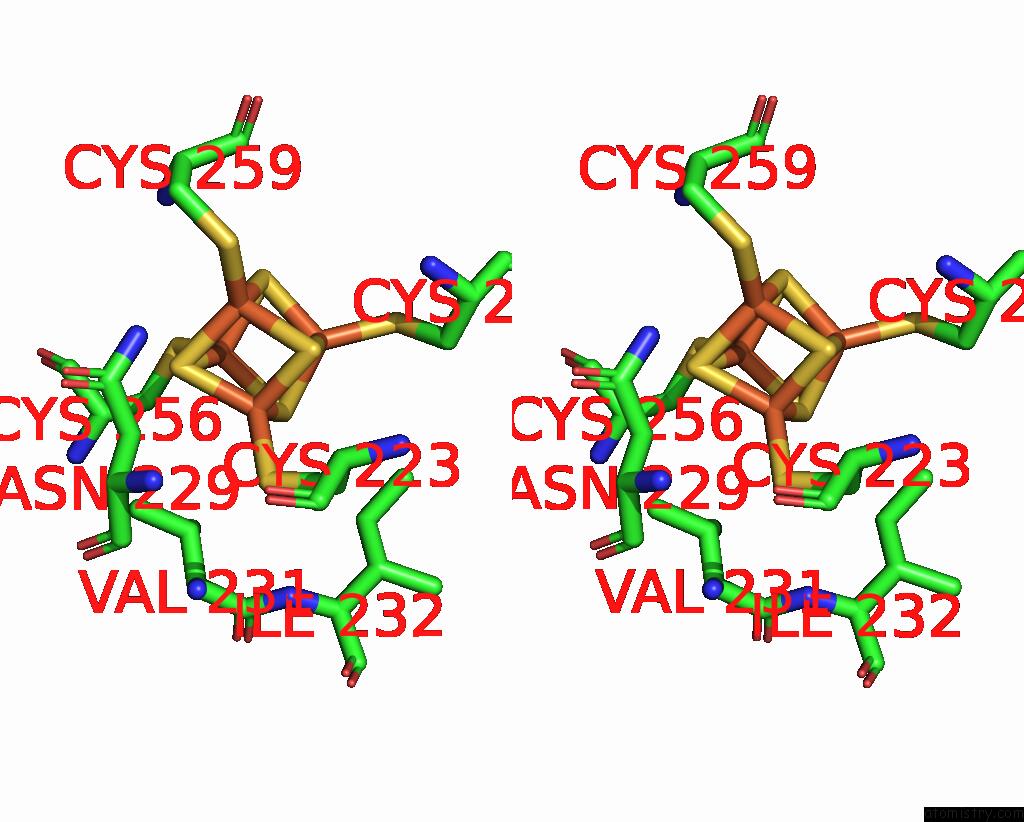
Iron binding site 8 out of 20 in 7vw6
Go back to
Iron binding site 8 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
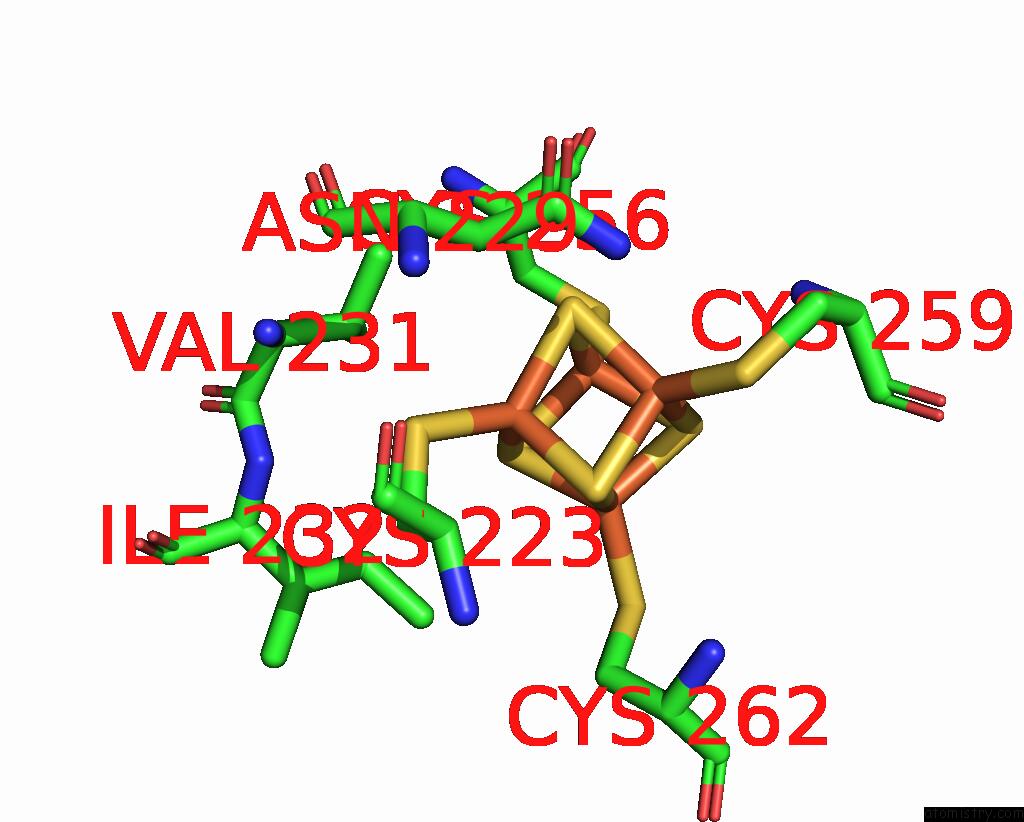
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
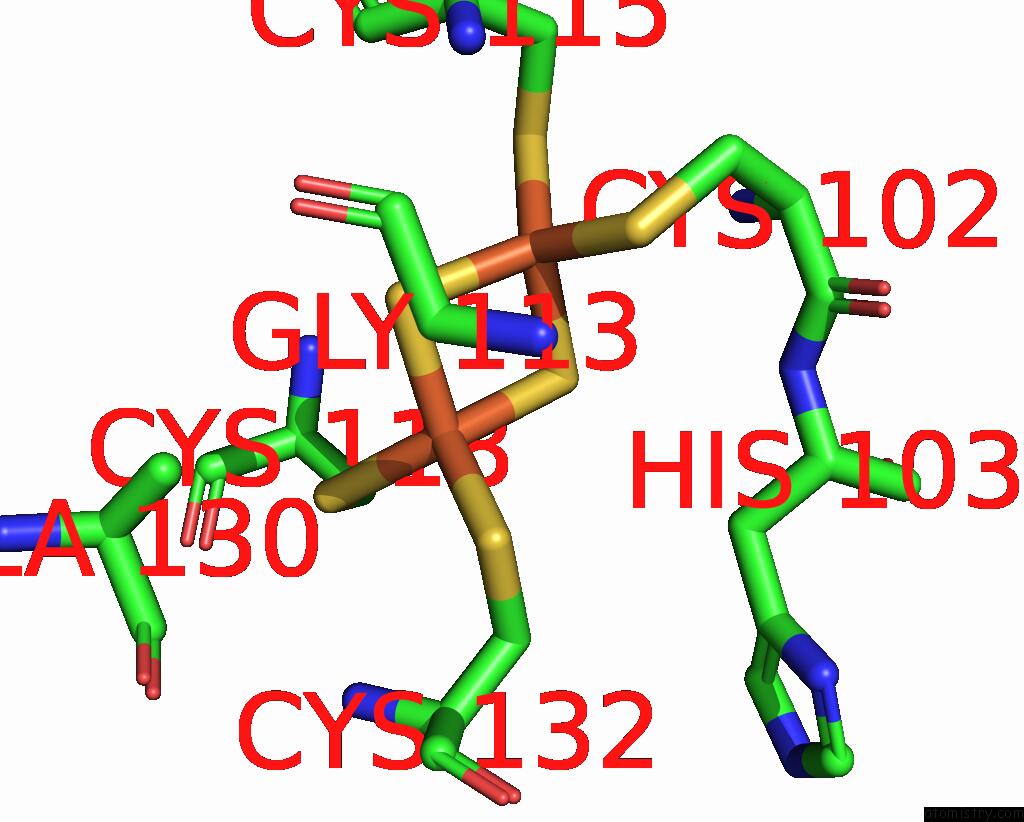
Iron binding site 9 out of 20 in 7vw6
Go back to
Iron binding site 9 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
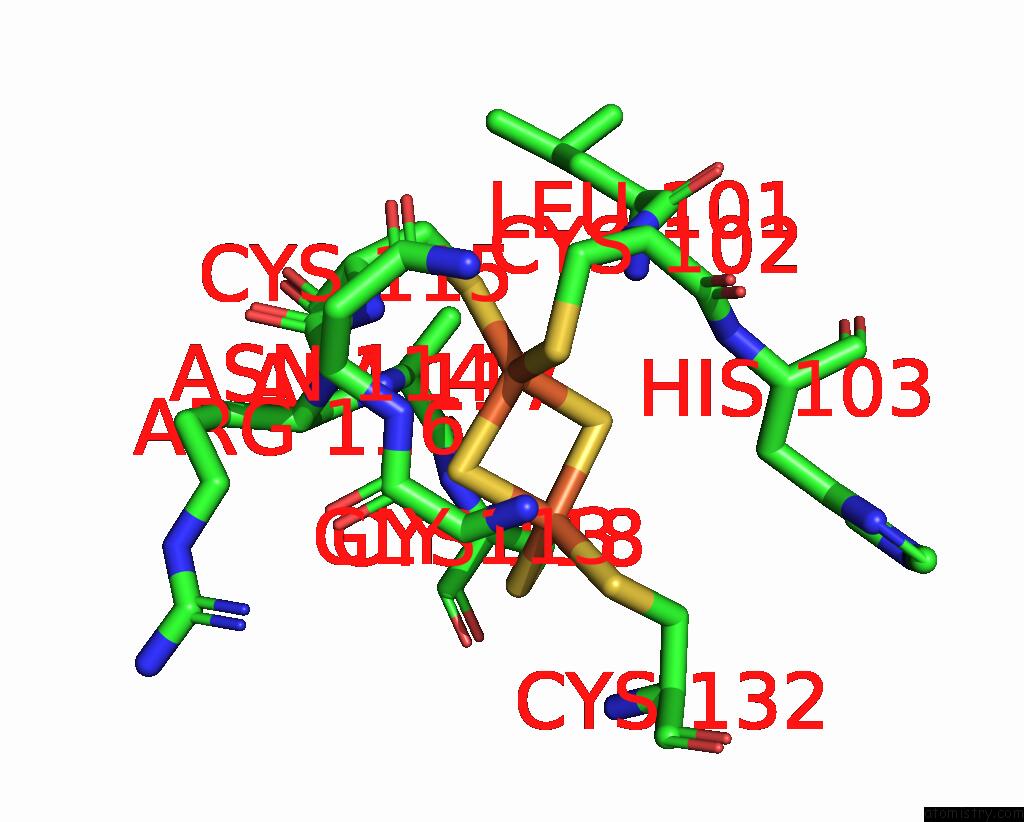
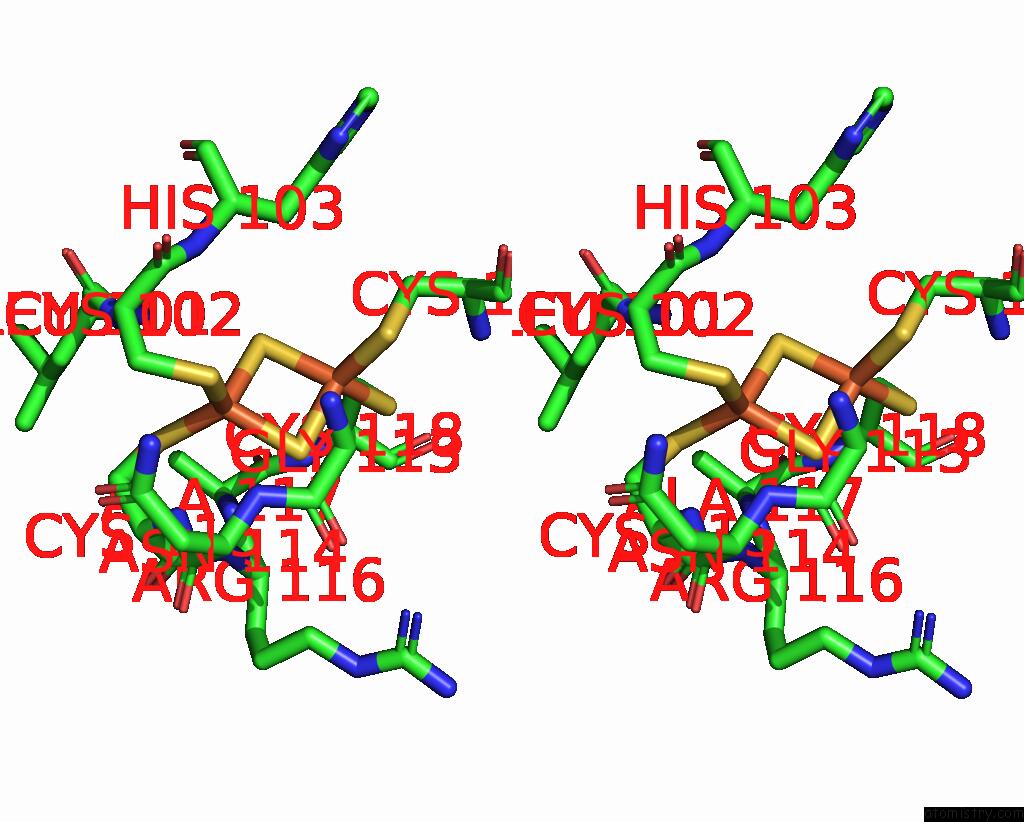
Iron binding site 10 out of 20 in 7vw6
Go back to
Iron binding site 10 out
of 20 in the Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Cryo-Em Structure of Formate Dehydrogenase 1 From Methylorubrum Extorquens AM1 within 5.0Å range:
|
Reference:
T.Yoshikawa,
F.Makino,
T.Miyata,
Y.Suzuki,
H.Tanaka,
K.Namba,
K.Kano,
K.Sowa,
Y.Kitazumi,
O.Shirai.
Multiple Electron Transfer Pathways of Tungsten-Containing Formate Dehydrogenase in Direct Electron Transfer-Type Bioelectrocatalysis. Chem.Commun.(Camb.) V. 58 6478 2022.
ISSN: ESSN 1364-548X
PubMed: 35535582
DOI: 10.1039/D2CC01541B
Page generated: Thu Aug 7 07:48:52 2025
ISSN: ESSN 1364-548X
PubMed: 35535582
DOI: 10.1039/D2CC01541B
Last articles
Mg in 1JB0Mg in 1IZL
Mg in 1JBZ
Mg in 1JBW
Mg in 1JBV
Mg in 1JBK
Mg in 1JAX
Mg in 1JAH
Mg in 1J97
Mg in 1J9J