Iron »
PDB 7wdh-7xgy »
7wy4 »
Iron in PDB 7wy4: Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene
Enzymatic activity of Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene
All present enzymatic activity of Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene:
1.14.14.1; 1.6.2.4;
1.14.14.1; 1.6.2.4;
Protein crystallography data
The structure of Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene, PDB code: 7wy4
was solved by
K.Suzuki,
J.K.Stanfield,
Y.Shisaka,
K.Omura,
C.Kasai,
H.Sugimoto,
O.Shoji,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.22 / 1.45 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.97, 126.65, 148.75, 90, 90, 90 |
| R / Rfree (%) | 14.3 / 17.9 |
Other elements in 7wy4:
The structure of Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene
(pdb code 7wy4). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene, PDB code: 7wy4:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene, PDB code: 7wy4:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 7wy4
Go back to
Iron binding site 1 out
of 4 in the Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene
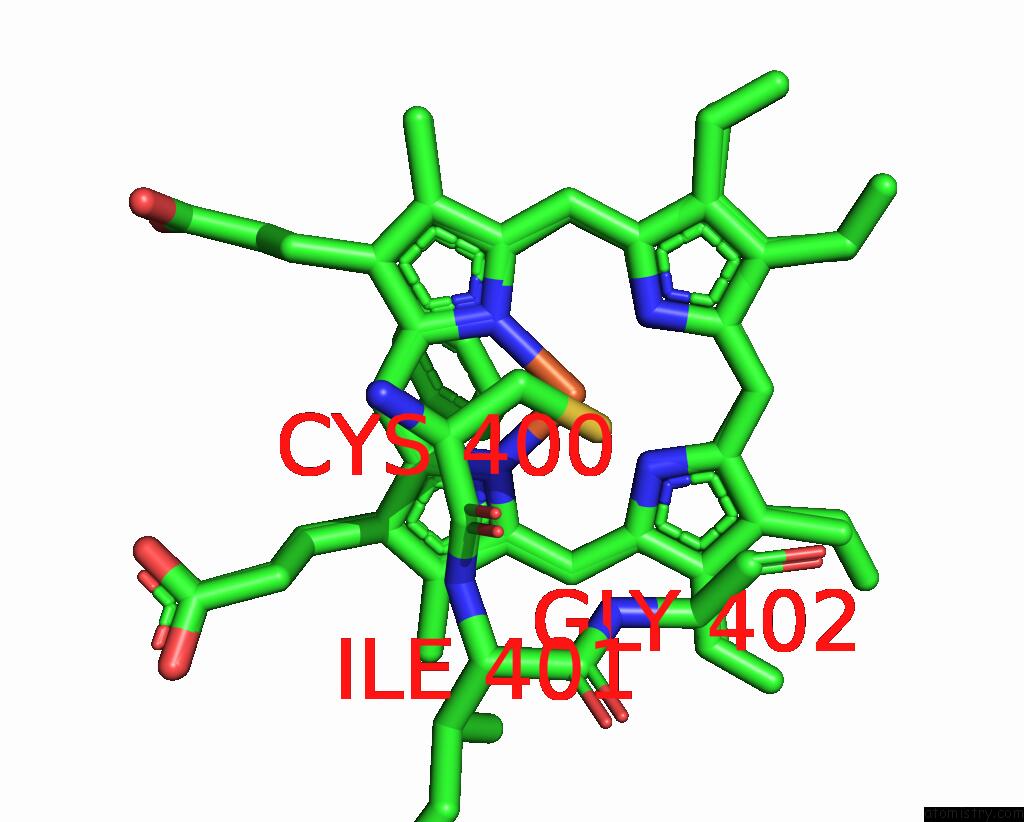
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene within 5.0Å range:
|
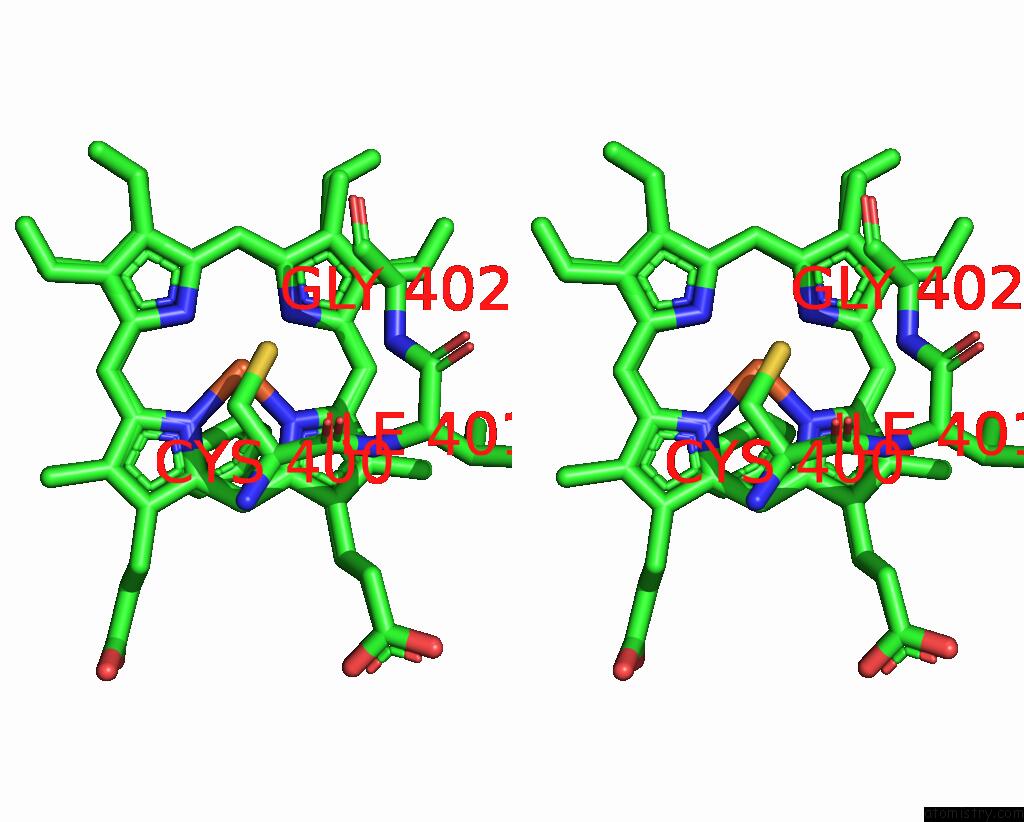
Iron binding site 2 out of 4 in 7wy4
Go back to
Iron binding site 2 out
of 4 in the Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene within 5.0Å range:
|
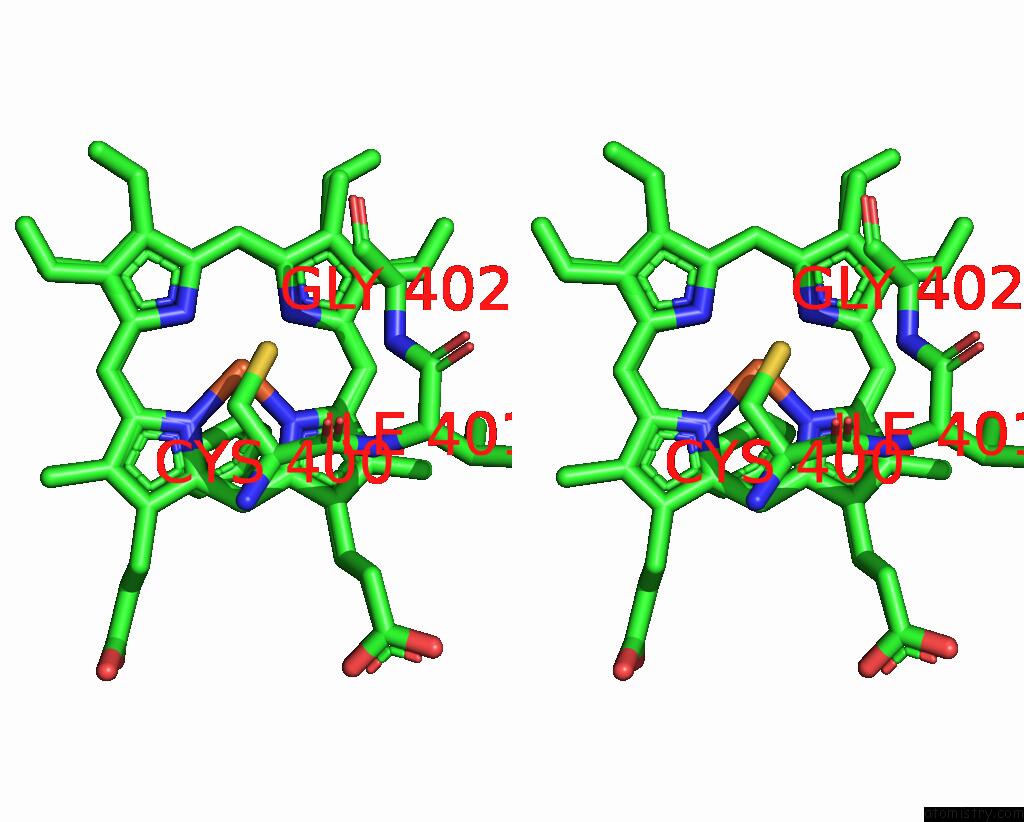
Iron binding site 3 out of 4 in 7wy4
Go back to
Iron binding site 3 out
of 4 in the Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene within 5.0Å range:
|
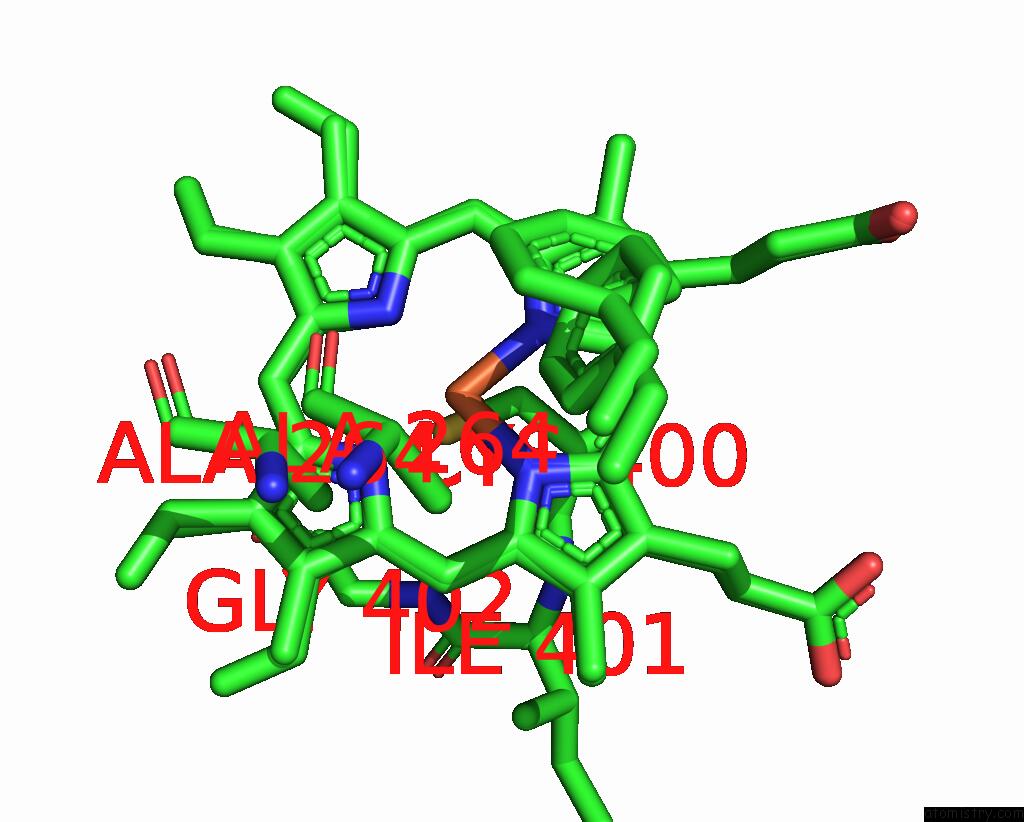
Iron binding site 4 out of 4 in 7wy4
Go back to
Iron binding site 4 out
of 4 in the Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene
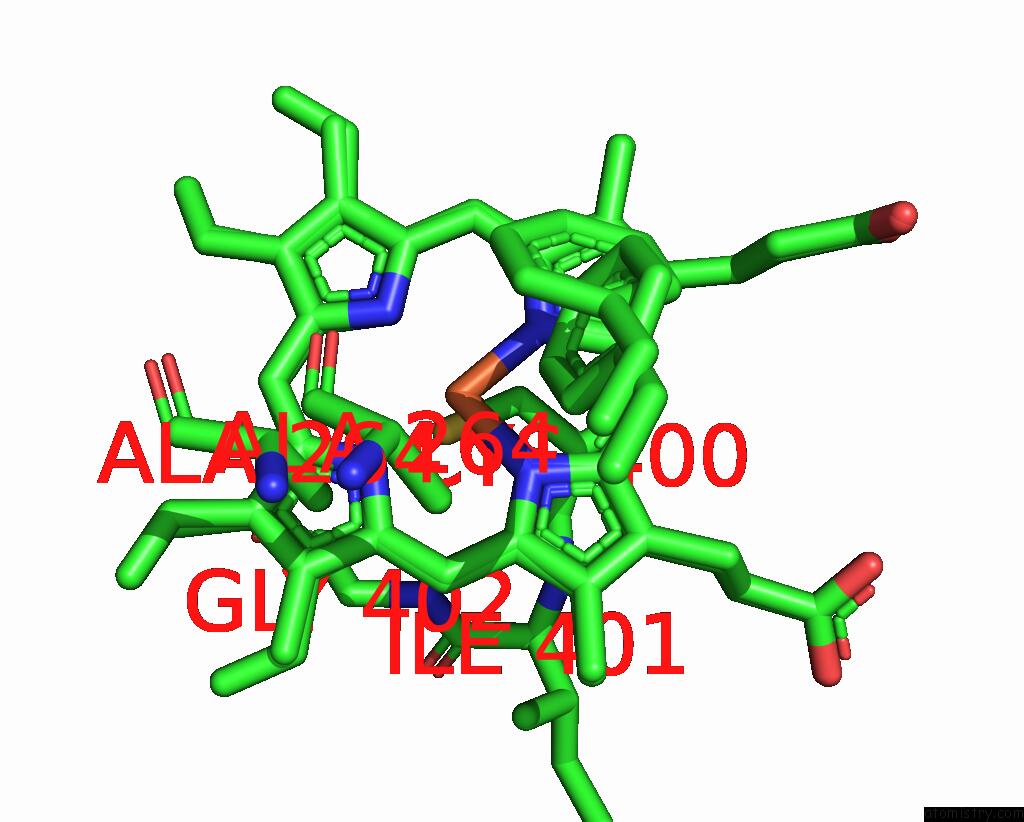
Mono view
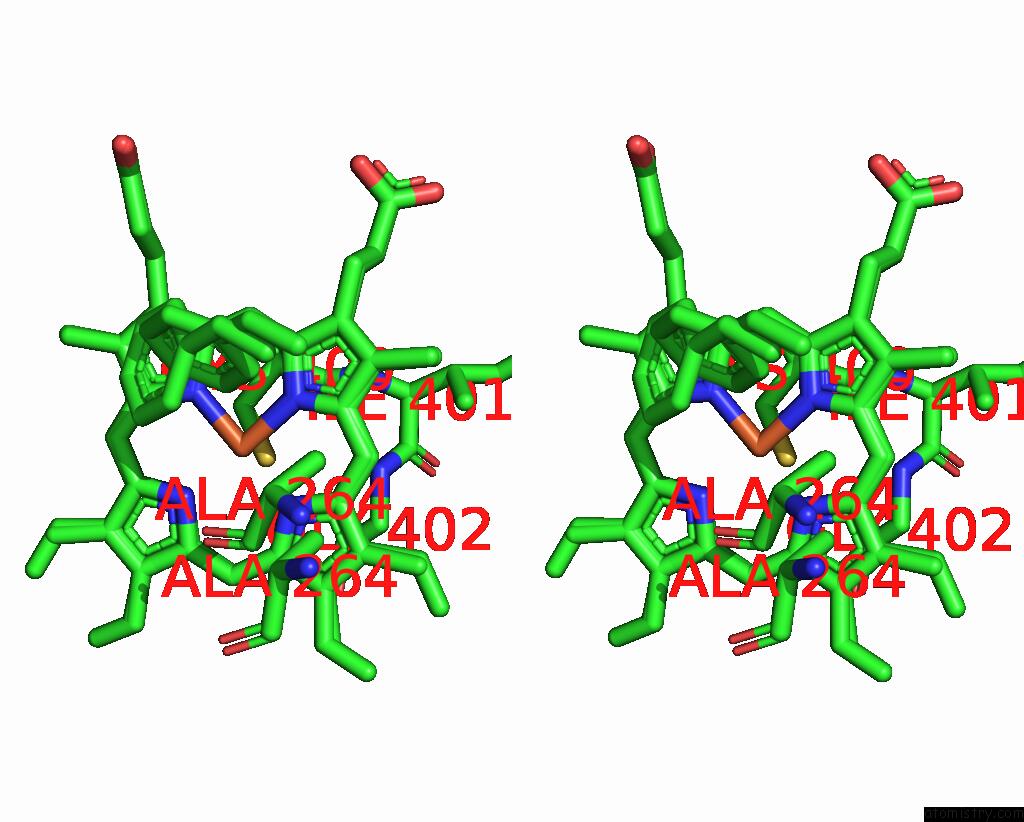
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Structure of the CYP102A1 F87A Haem Domain with N-Enanthyl-L-Prolyl-L- Phenylalanine in Complex with Styrene within 5.0Å range:
|
Reference:
K.Suzuki,
J.K.Stanfield,
K.Omura,
Y.Shisaka,
S.Ariyasu,
C.Kasai,
Y.Aiba,
H.Sugimoto,
O.Shoji.
A Compound I Mimic Reveals the Transient Active Species of A Cytochrome P450 Enzyme: Insight Into the Stereoselectivity of P450-Catalysed Oxidations. Angew.Chem.Int.Ed.Engl. 2022.
ISSN: ESSN 1521-3773
PubMed: 36519803
DOI: 10.1002/ANIE.202215706
Page generated: Thu Aug 7 09:53:49 2025
ISSN: ESSN 1521-3773
PubMed: 36519803
DOI: 10.1002/ANIE.202215706
Last articles
Mg in 1VQ4Mg in 1VPA
Mg in 1VPE
Mg in 1VOM
Mg in 1VMA
Mg in 1VMK
Mg in 1VM9
Mg in 1VCR
Mg in 1VLB
Mg in 1VKP