Iron »
PDB 7xgy-7y9j »
7xqw »
Iron in PDB 7xqw: Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
Enzymatic activity of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
All present enzymatic activity of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1):
1.17.1.9; 1.2.1.2;
1.17.1.9; 1.2.1.2;
Other elements in 7xqw:
The structure of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) also contains other interesting chemical elements:
| Tungsten | (W) | 1 atom |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20;Binding sites:
The binding sites of Iron atom in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) (pdb code 7xqw). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 20 binding sites of Iron where determined in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1), PDB code: 7xqw:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 20 in 7xqw
Go back to
Iron binding site 1 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
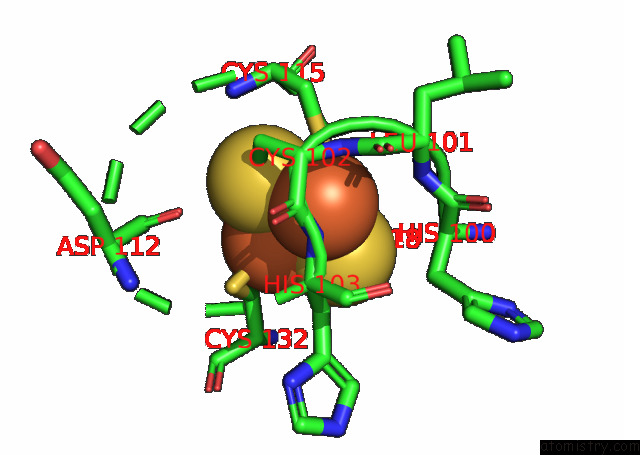
Mono view
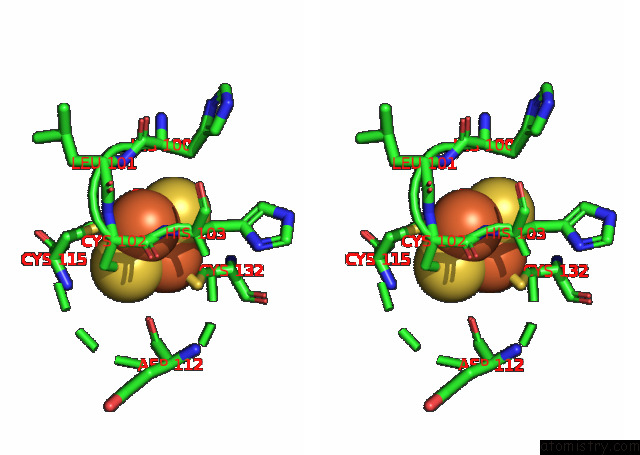
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 2 out of 20 in 7xqw
Go back to
Iron binding site 2 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
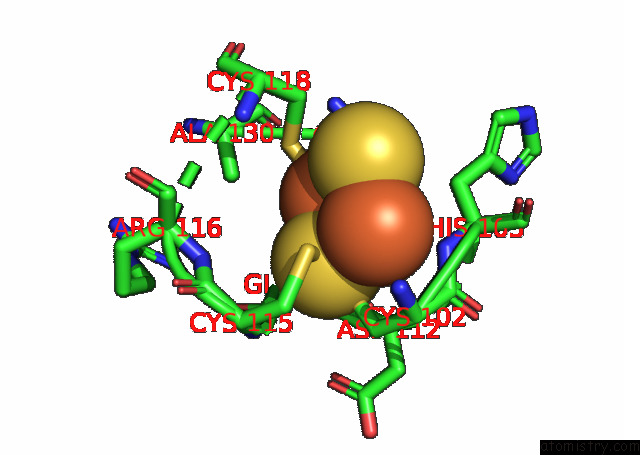
Mono view
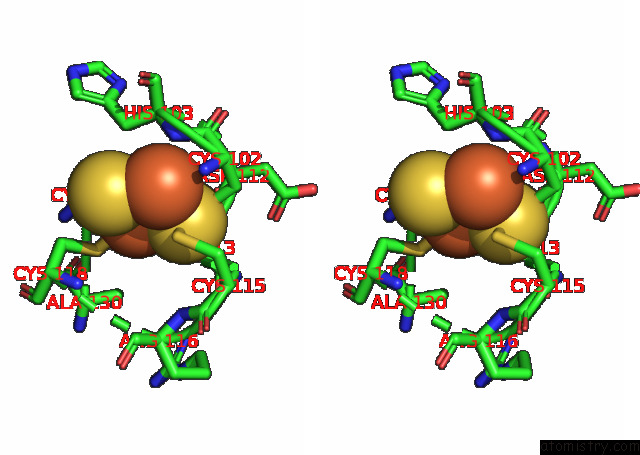
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 3 out of 20 in 7xqw
Go back to
Iron binding site 3 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
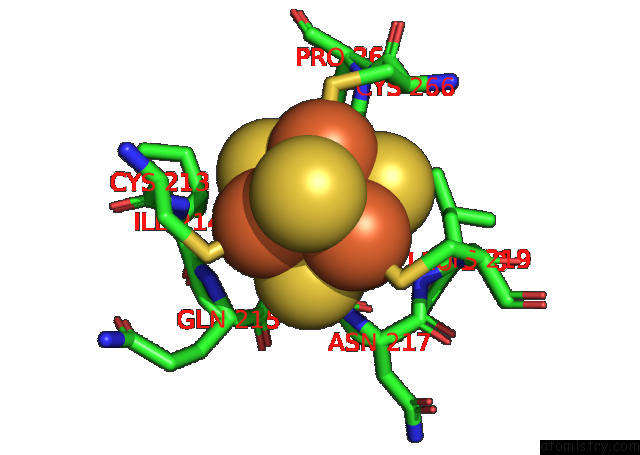
Mono view
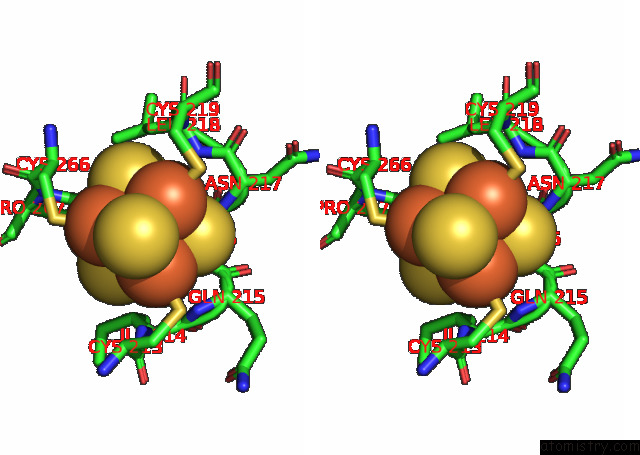
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 4 out of 20 in 7xqw
Go back to
Iron binding site 4 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
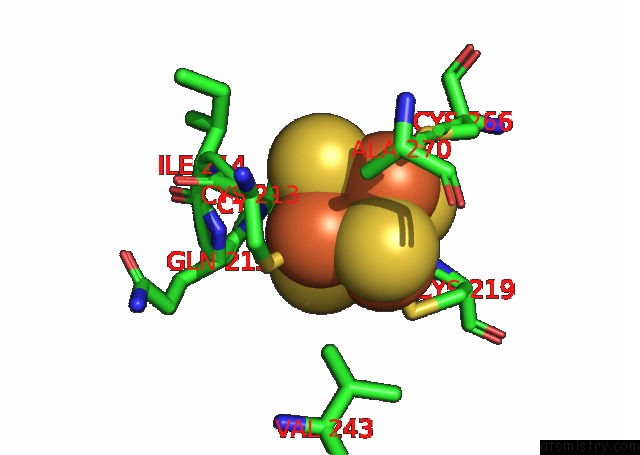
Mono view
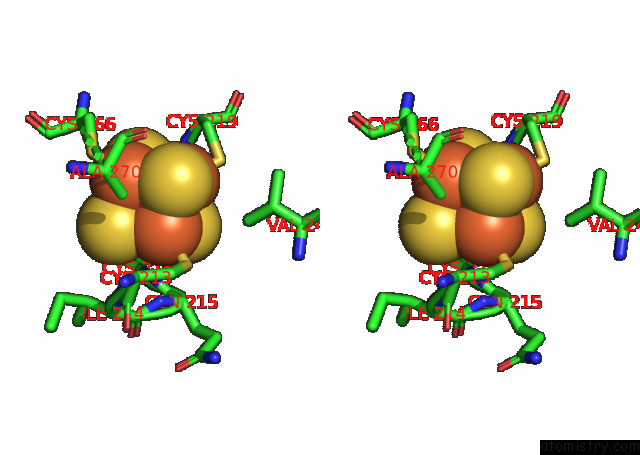
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 5 out of 20 in 7xqw
Go back to
Iron binding site 5 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
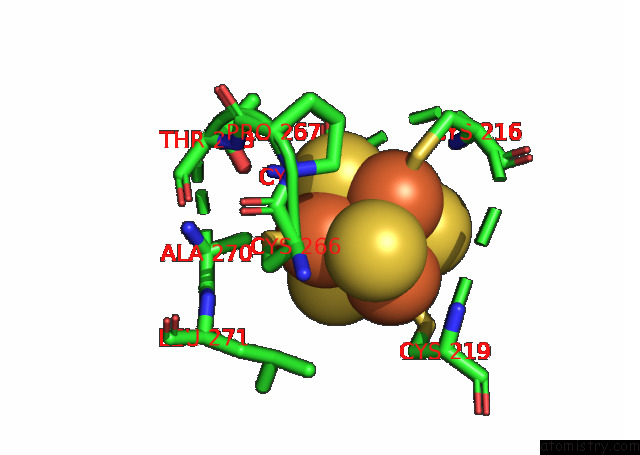
Mono view
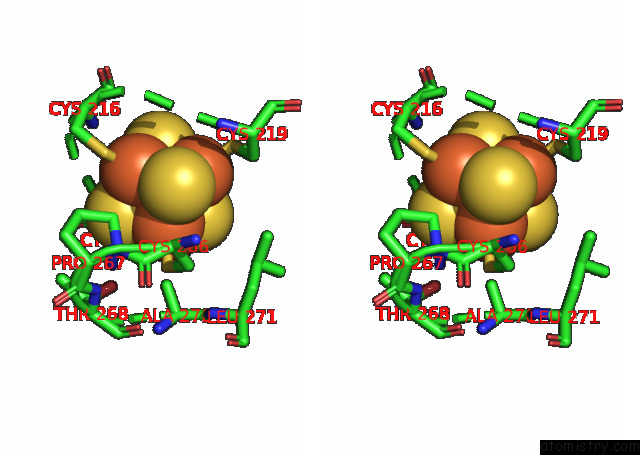
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 6 out of 20 in 7xqw
Go back to
Iron binding site 6 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
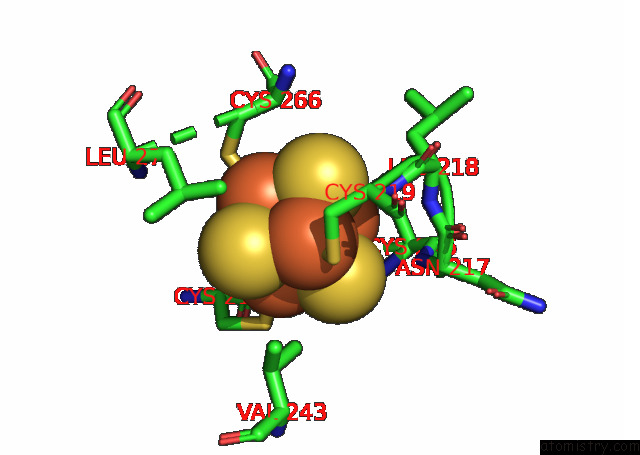
Mono view
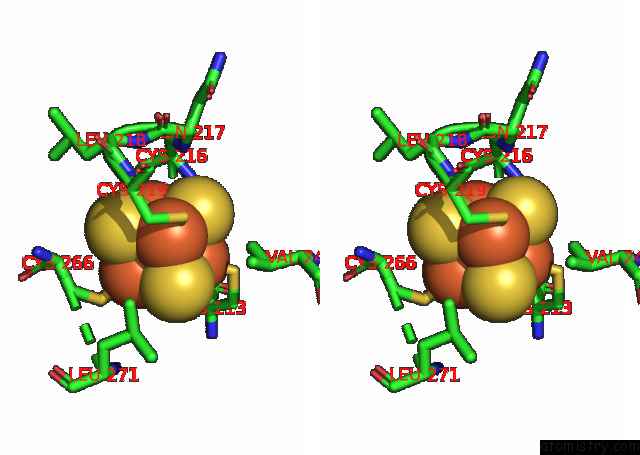
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 7 out of 20 in 7xqw
Go back to
Iron binding site 7 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
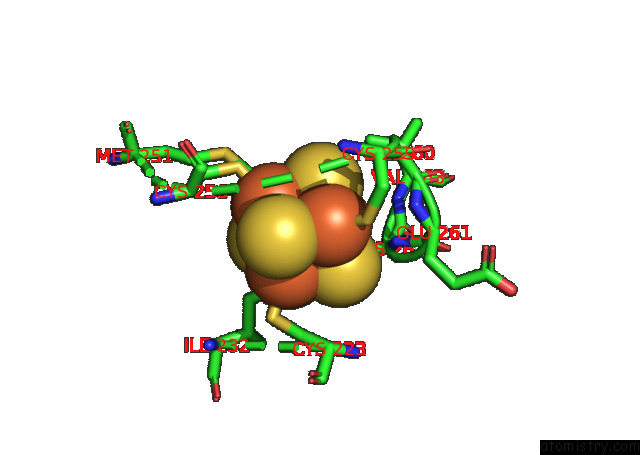
Mono view
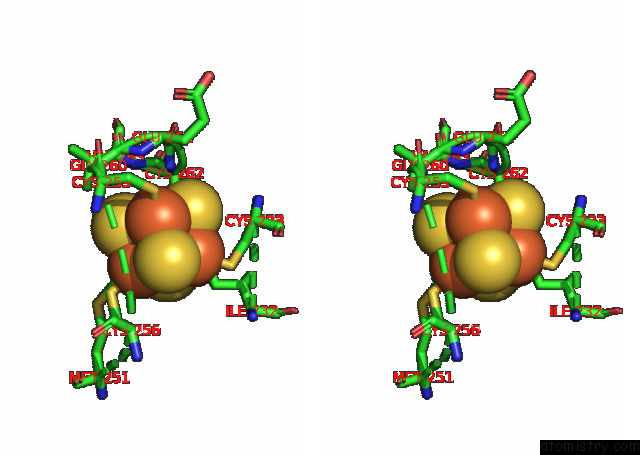
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 8 out of 20 in 7xqw
Go back to
Iron binding site 8 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
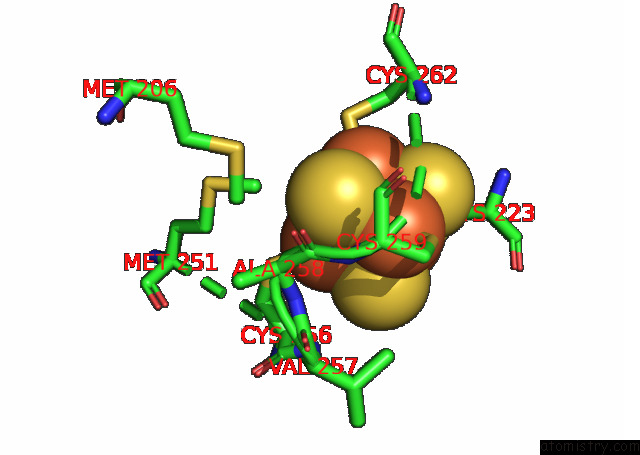
Mono view
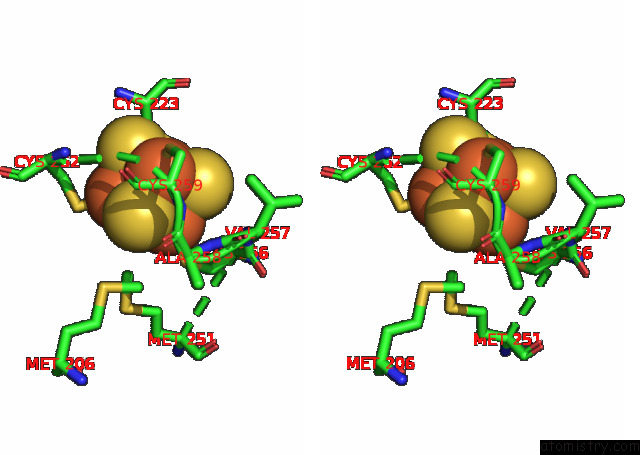
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 9 out of 20 in 7xqw
Go back to
Iron binding site 9 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
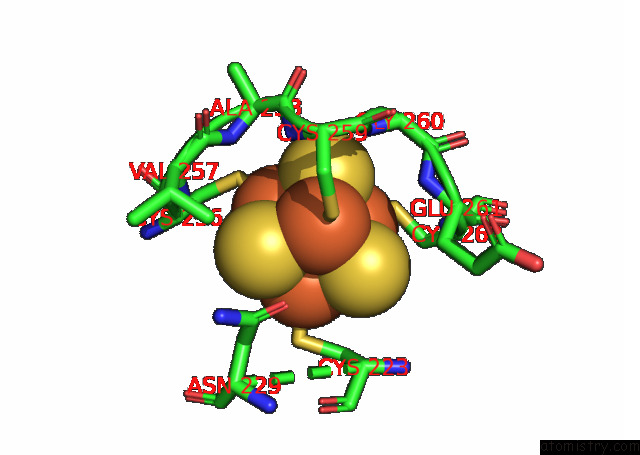
Mono view
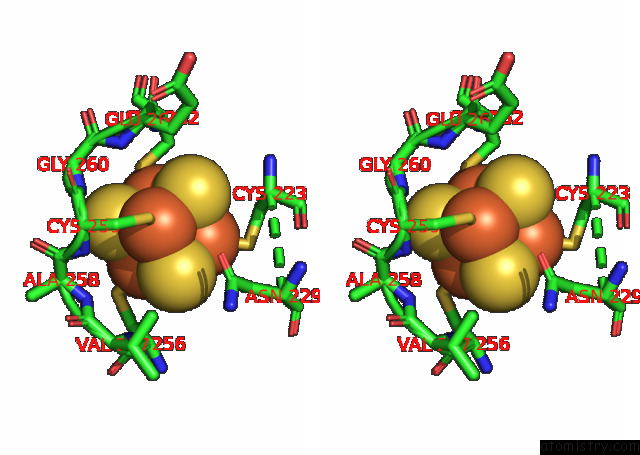
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Iron binding site 10 out of 20 in 7xqw
Go back to
Iron binding site 10 out
of 20 in the Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1)
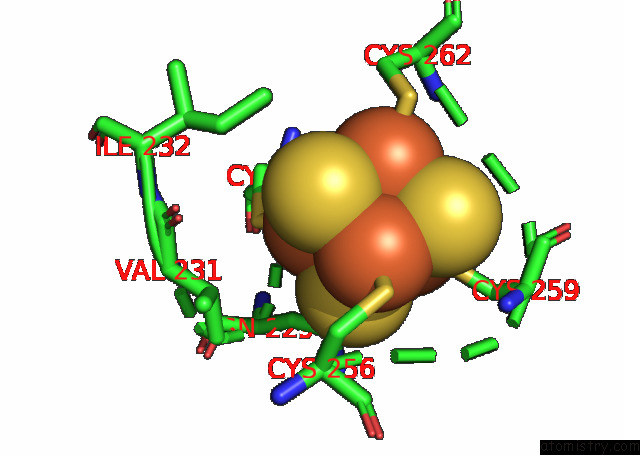
Mono view
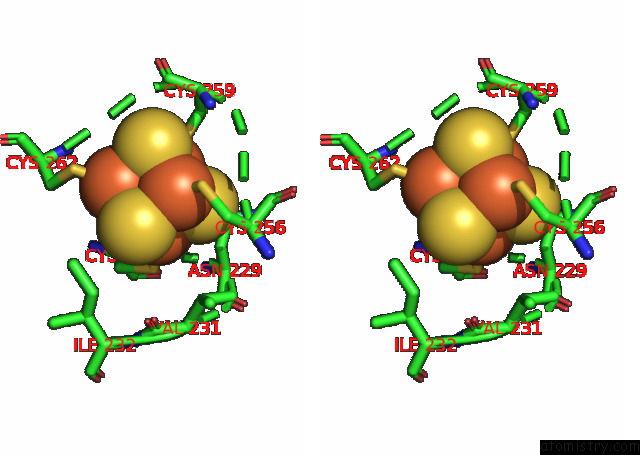
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Formate Dehydrogenase (Fdh) From Methylobacterium Extorquens AM1 (MEFDH1) within 5.0Å range:
|
Reference:
B.W.Jeon,
Y.Y.Heo,
J.Park,
S.H.Roh,
H.H.Lee,
Y.H.Kim.
Enzymatic Conversion of CO2 in Real Flue Gas to Molar-Scale Formate To Be Published.
Page generated: Fri Aug 9 11:08:37 2024
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF