Iron »
PDB 8abg-8aq0 »
8ahx »
Iron in PDB 8ahx: Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 18;Binding sites:
The binding sites of Iron atom in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii (pdb code 8ahx). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 18 binding sites of Iron where determined in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii, PDB code: 8ahx:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
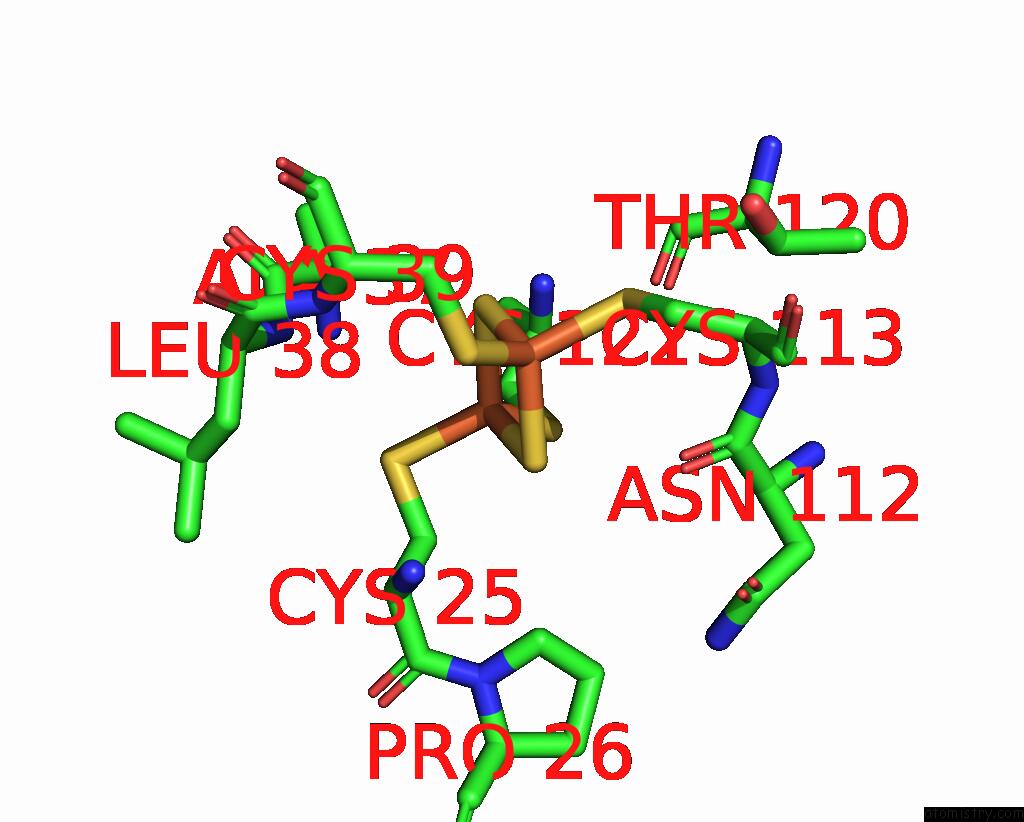
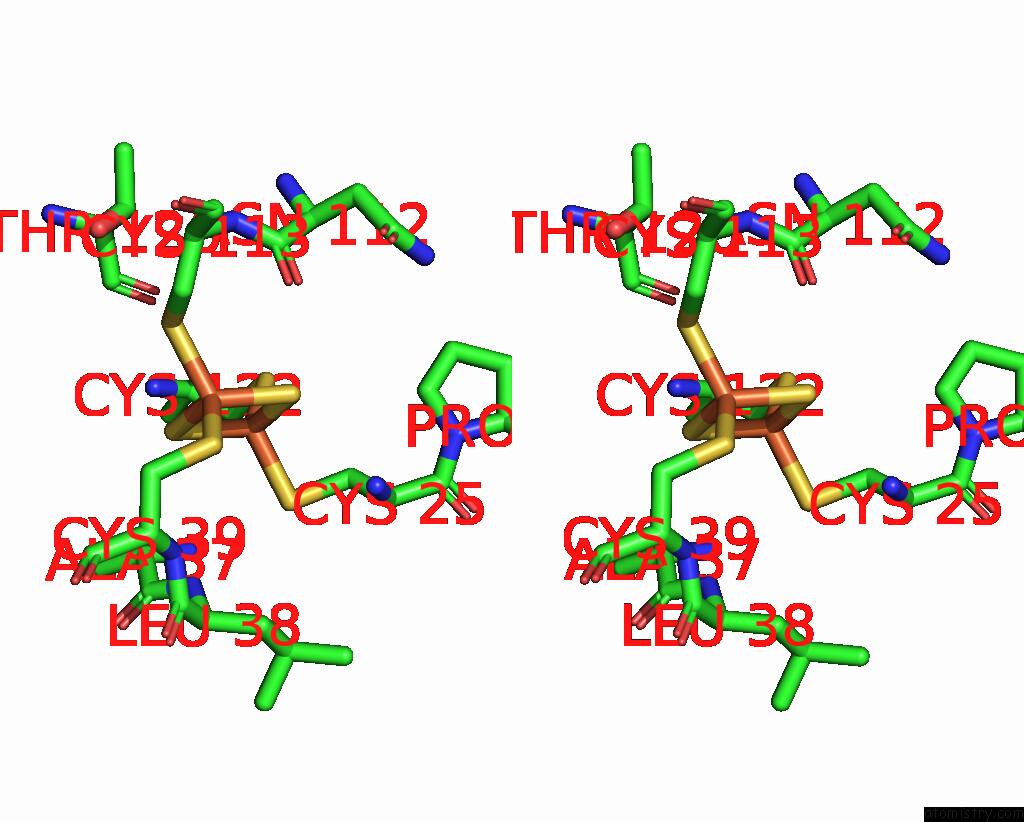
Iron binding site 1 out of 18 in 8ahx
Go back to
Iron binding site 1 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 2 out of 18 in 8ahx
Go back to
Iron binding site 2 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
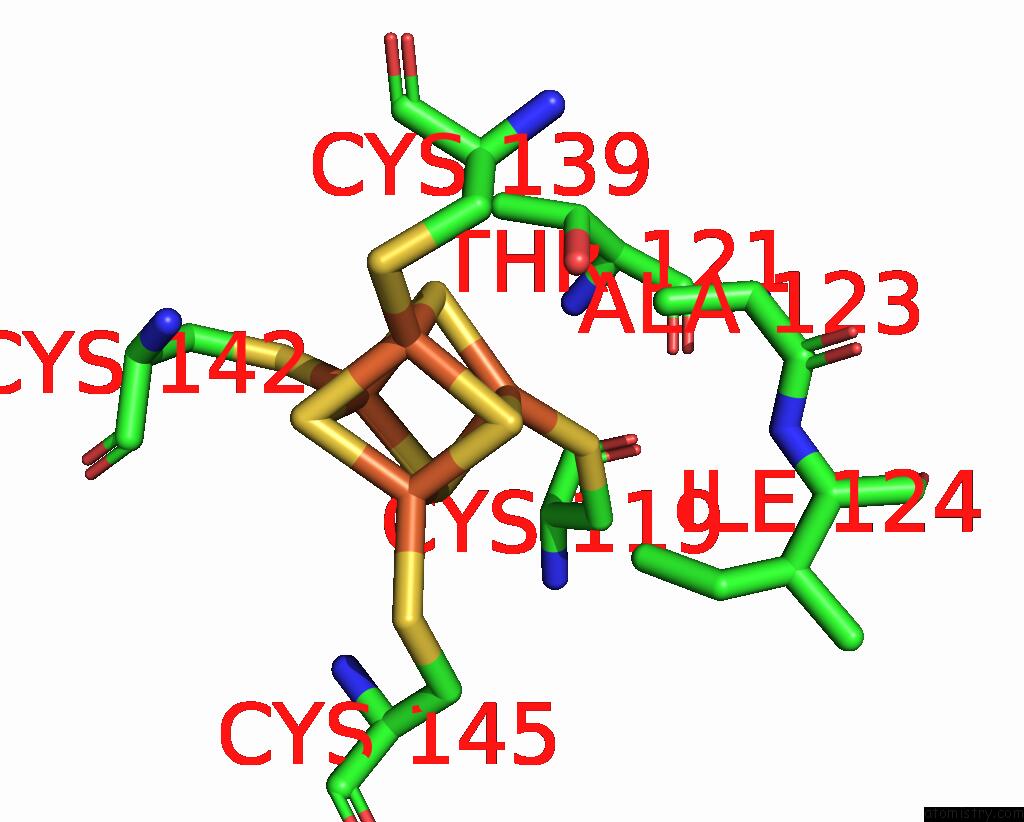
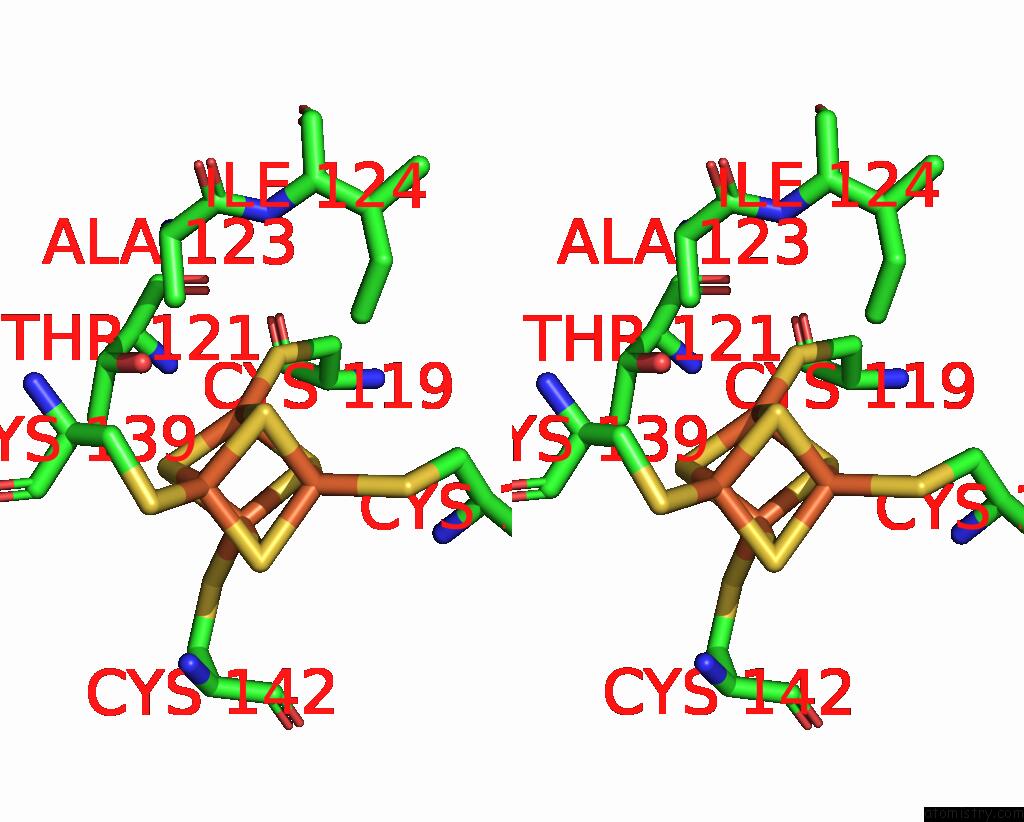
Iron binding site 3 out of 18 in 8ahx
Go back to
Iron binding site 3 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
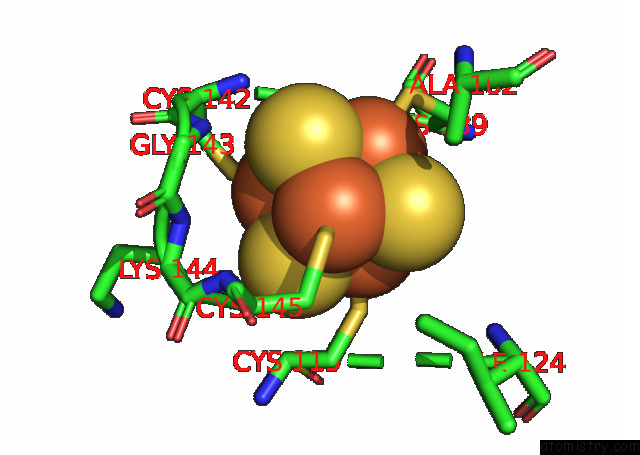
Iron binding site 4 out of 18 in 8ahx
Go back to
Iron binding site 4 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 5 out of 18 in 8ahx
Go back to
Iron binding site 5 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
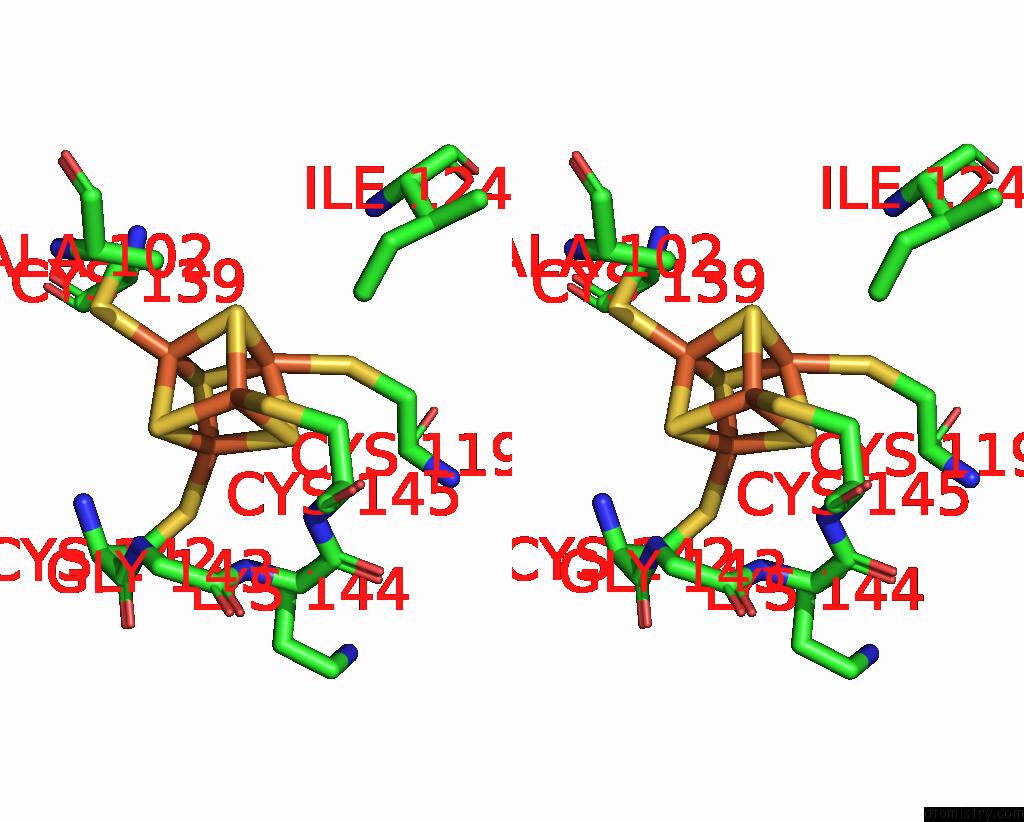
Iron binding site 6 out of 18 in 8ahx
Go back to
Iron binding site 6 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
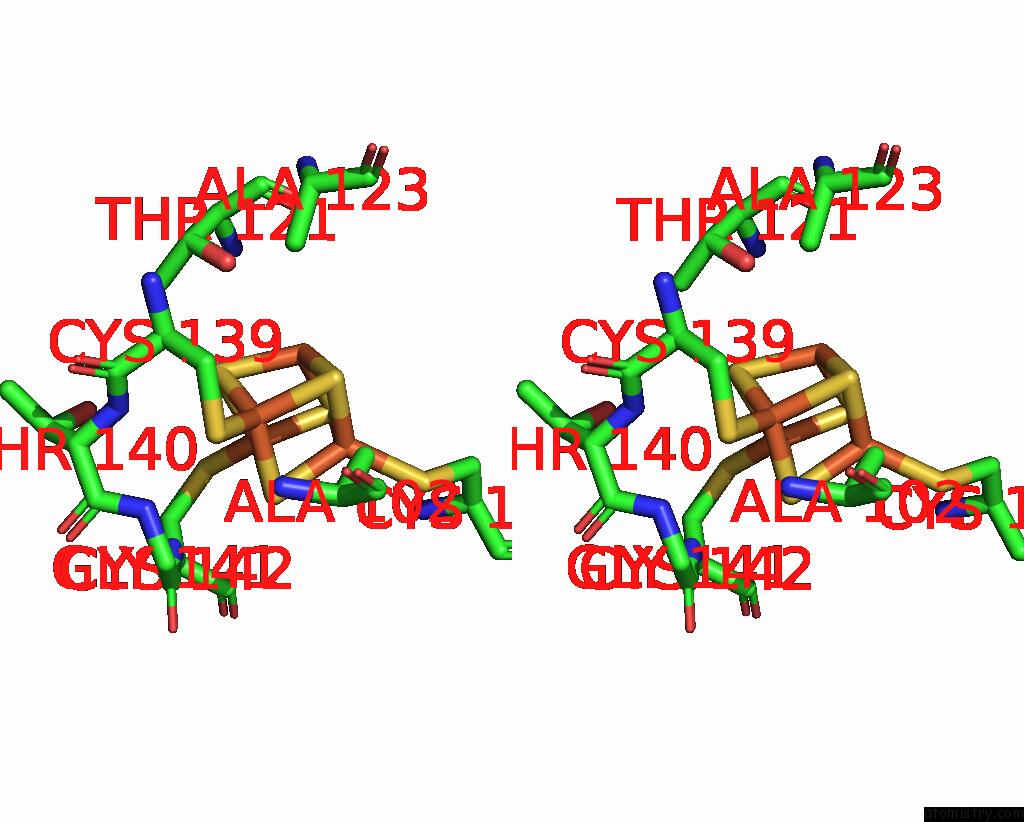
Iron binding site 7 out of 18 in 8ahx
Go back to
Iron binding site 7 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 8 out of 18 in 8ahx
Go back to
Iron binding site 8 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii
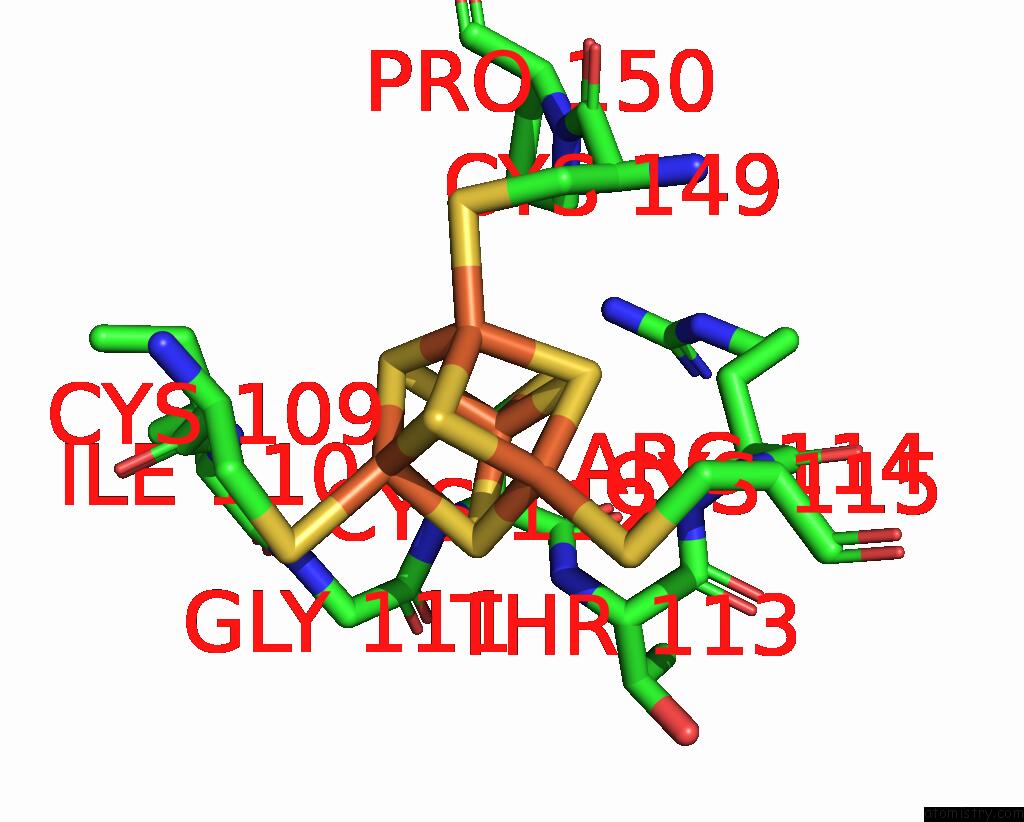
Mono view
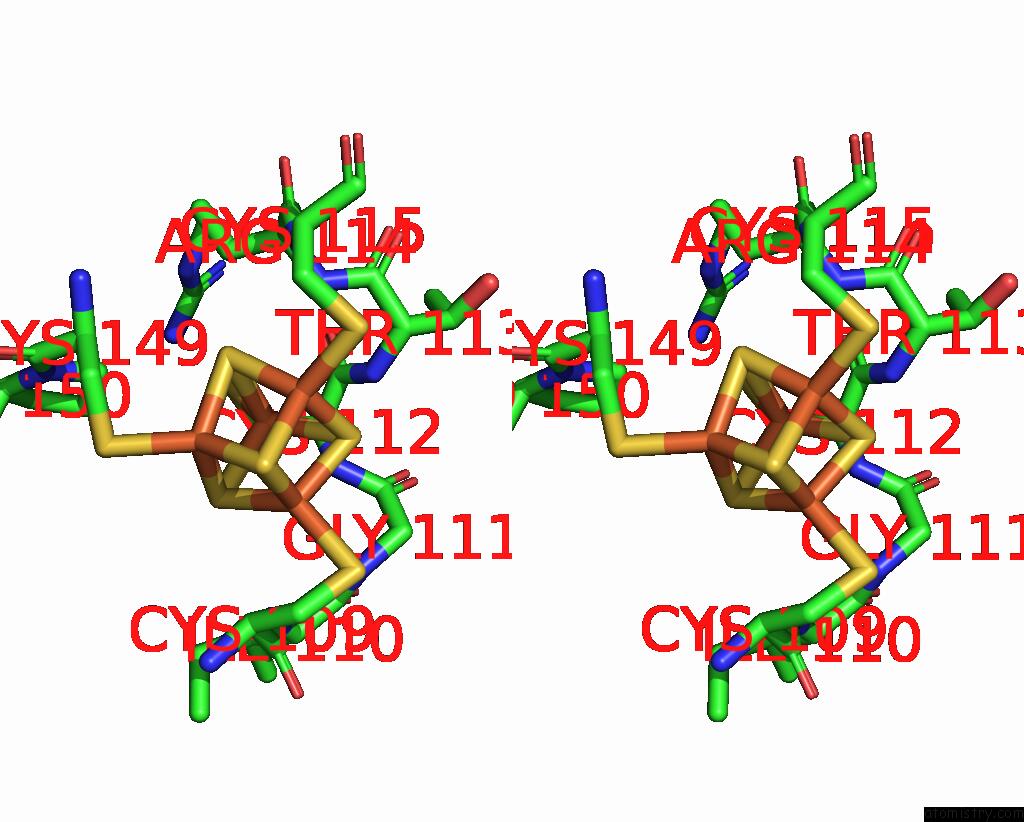
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
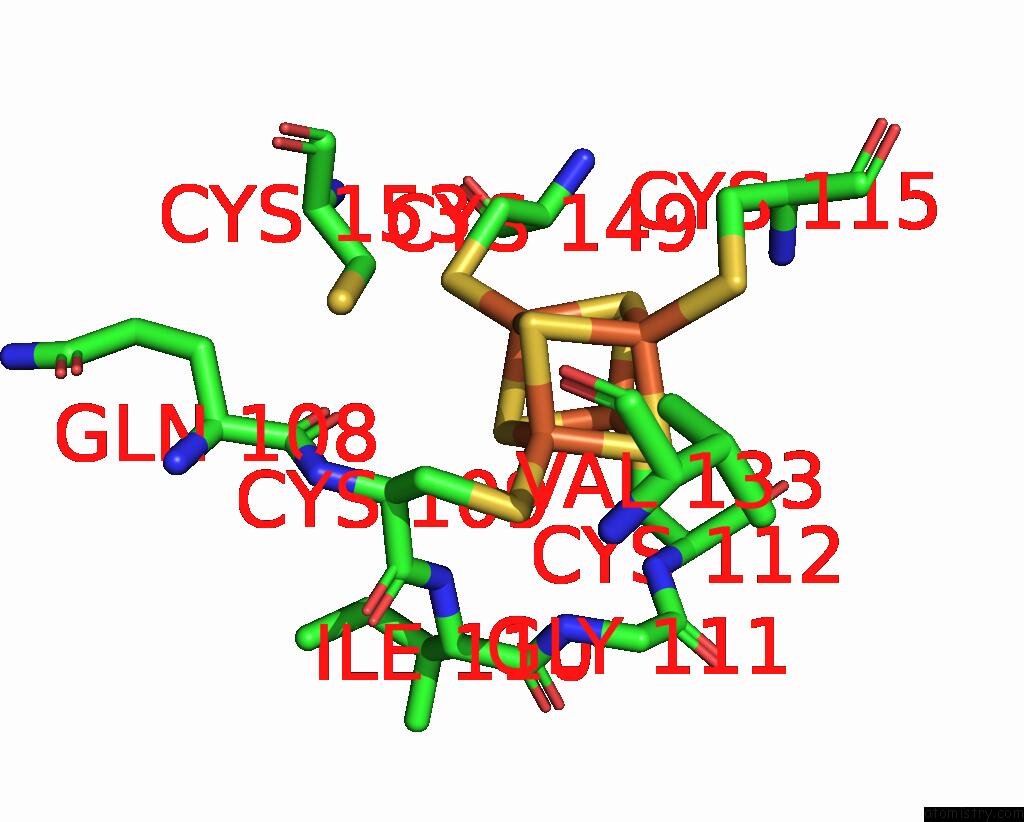
Iron binding site 9 out of 18 in 8ahx
Go back to
Iron binding site 9 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii

Mono view
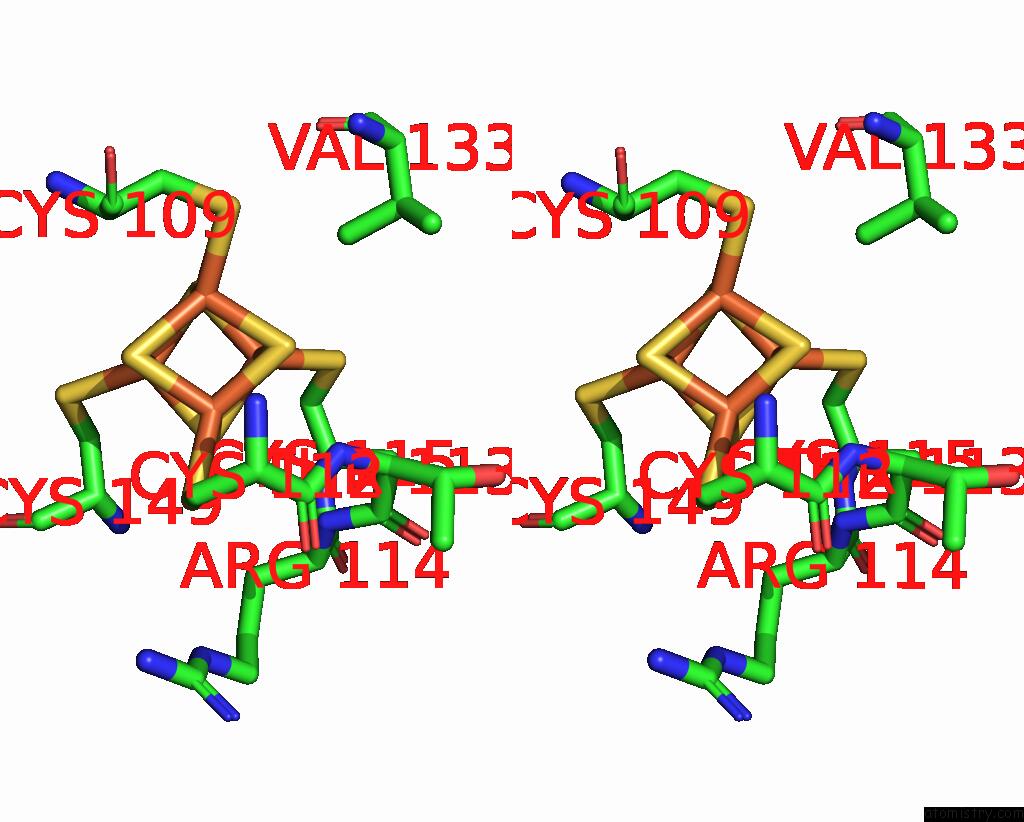
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
Iron binding site 10 out of 18 in 8ahx
Go back to
Iron binding site 10 out
of 18 in the Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii
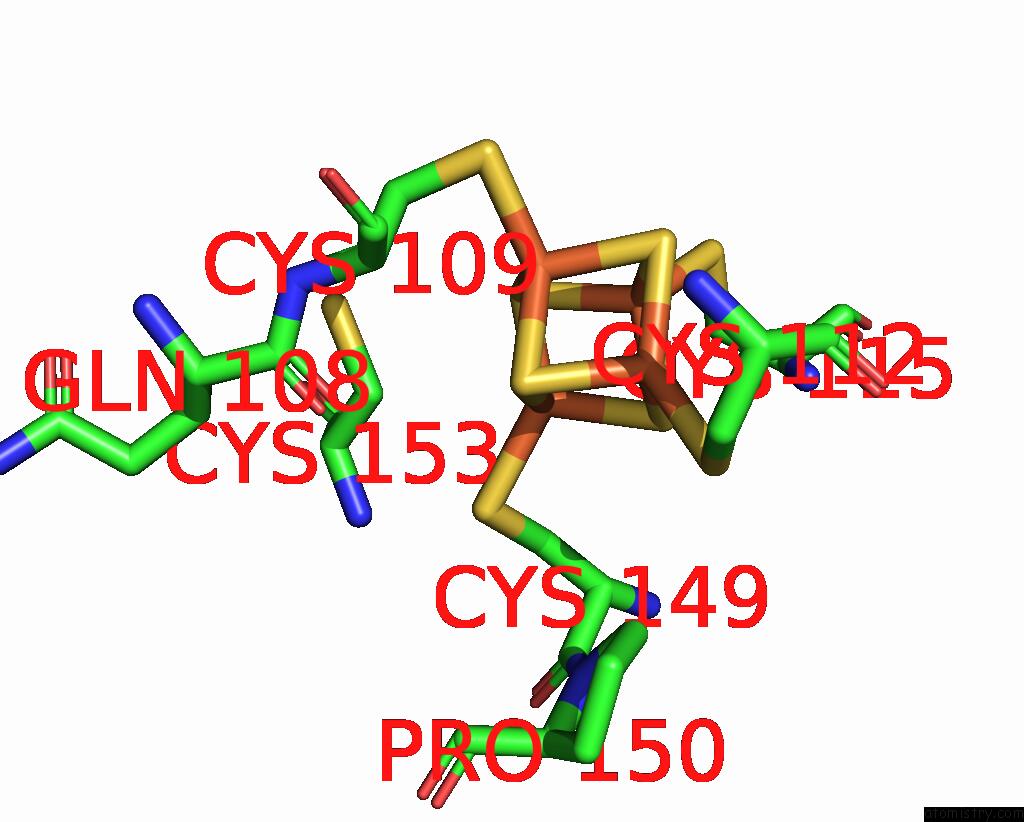
Mono view
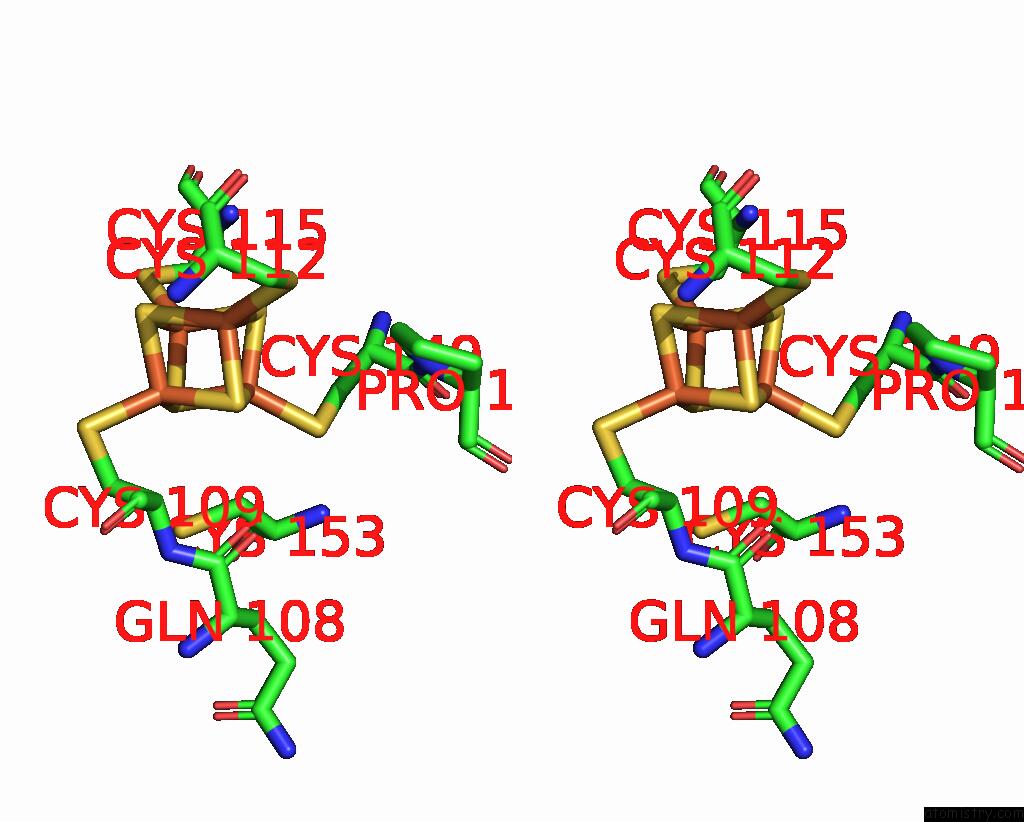
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Cryo-Em Structure of the Nitrogen-Fixation Associated Nadh:Ferredoxin Oxidoreductase Rnf From Azotobacter Vinelandii within 5.0Å range:
|
Reference:
L.Zhang,
O.Einsle.
Architecture of the Nadh:Ferredoxin Oxidoreductase Rnf That Drives Biological Nitrogen Fixation To Be Published.
Page generated: Fri Aug 9 17:27:21 2024
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW