Iron »
PDB 8e3u-8eqm »
8eko »
Iron in PDB 8eko: Sperm Whale Myoglobin Mutant L29H F33W F43H (F33W Cubmb)
Protein crystallography data
The structure of Sperm Whale Myoglobin Mutant L29H F33W F43H (F33W Cubmb), PDB code: 8eko
was solved by
A.P.Ledray,
S.Dwaraknath,
Y.Lu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.61 / 1.34 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 40.059, 48.07, 77.739, 90, 90, 90 |
| R / Rfree (%) | 13.9 / 16.4 |
Iron Binding Sites:
The binding sites of Iron atom in the Sperm Whale Myoglobin Mutant L29H F33W F43H (F33W Cubmb)
(pdb code 8eko). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Sperm Whale Myoglobin Mutant L29H F33W F43H (F33W Cubmb), PDB code: 8eko:
In total only one binding site of Iron was determined in the Sperm Whale Myoglobin Mutant L29H F33W F43H (F33W Cubmb), PDB code: 8eko:
Iron binding site 1 out of 1 in 8eko
Go back to
Iron binding site 1 out
of 1 in the Sperm Whale Myoglobin Mutant L29H F33W F43H (F33W Cubmb)
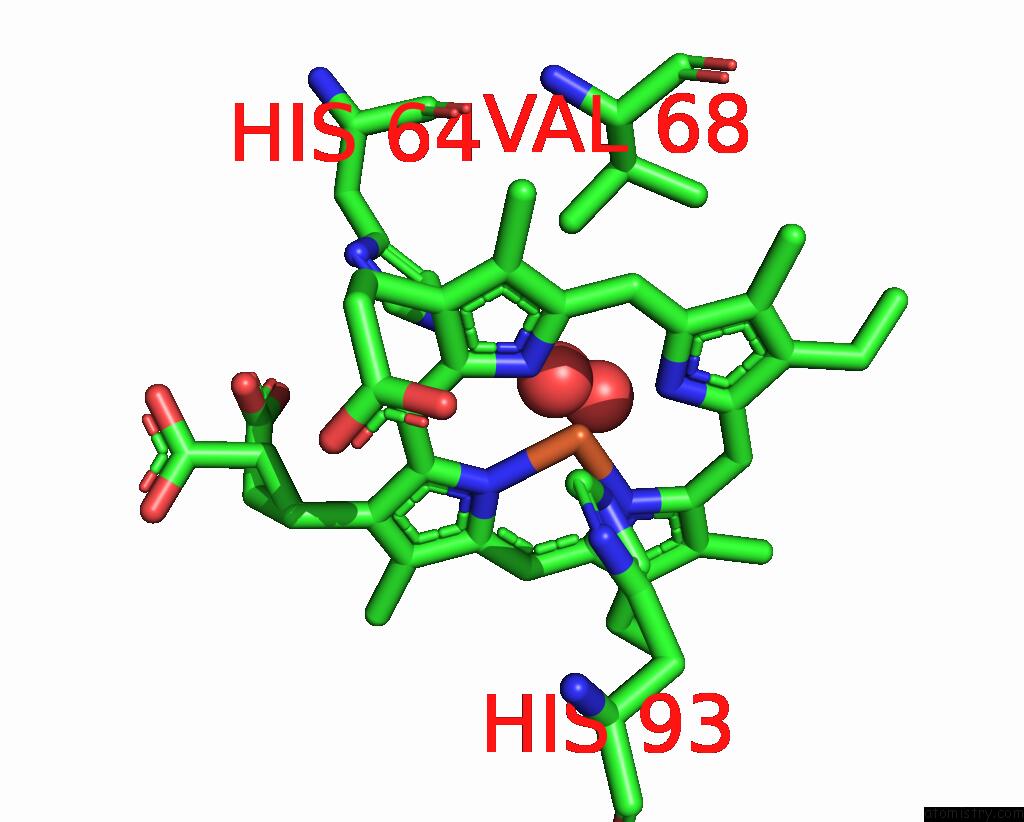
Mono view
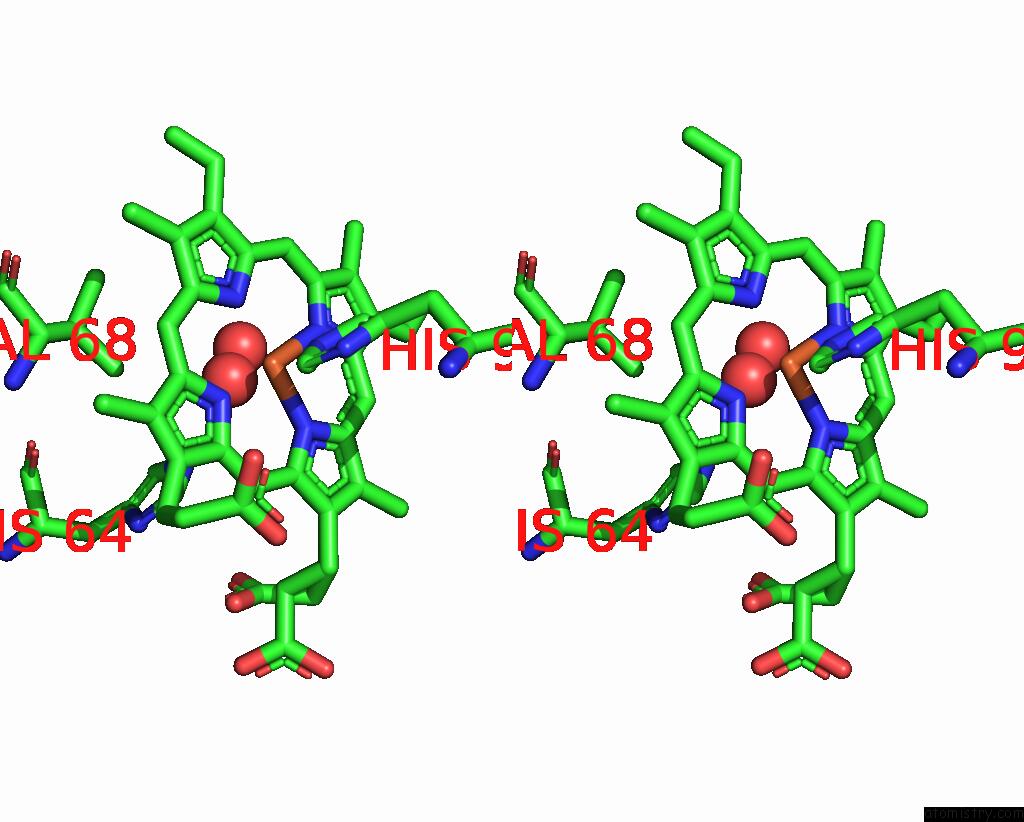
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Sperm Whale Myoglobin Mutant L29H F33W F43H (F33W Cubmb) within 5.0Å range:
|
Reference:
A.P.Ledray,
S.Dwaraknath,
K.Chakarawet,
M.R.Sponholtz,
C.Merchen,
C.Van Stappen,
G.Rao,
R.D.Britt,
Y.Lu.
Tryptophan Can Promote Oxygen Reduction to Water in A Biosynthetic Model of Heme Copper Oxidases. Biochemistry 2022.
ISSN: ISSN 0006-2960
PubMed: 36215733
DOI: 10.1021/ACS.BIOCHEM.2C00300
Page generated: Sat Aug 10 01:30:36 2024
ISSN: ISSN 0006-2960
PubMed: 36215733
DOI: 10.1021/ACS.BIOCHEM.2C00300
Last articles
Cl in 3UTACl in 3USC
Cl in 3UTU
Cl in 3USE
Cl in 3UT9
Cl in 3USS
Cl in 3USR
Cl in 3USP
Cl in 3USQ
Cl in 3URY