Iron »
PDB 8euh-8f9n »
8f6c »
Iron in PDB 8f6c: E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer
Enzymatic activity of E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer
All present enzymatic activity of E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer:
7.1.1.3;
7.1.1.3;
Other elements in 8f6c:
The structure of E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer also contains other interesting chemical elements:
| Copper | (Cu) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer
(pdb code 8f6c). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer, PDB code: 8f6c:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer, PDB code: 8f6c:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 8f6c
Go back to
Iron binding site 1 out
of 4 in the E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer

Mono view
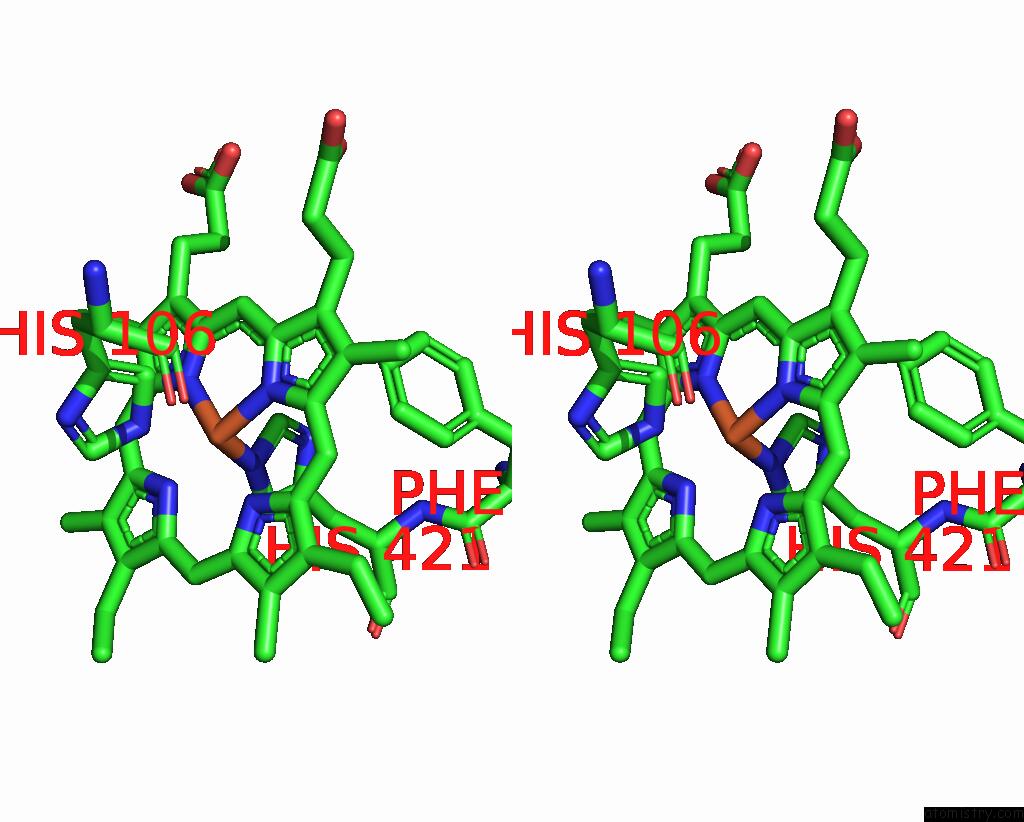
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer within 5.0Å range:
|
Iron binding site 2 out of 4 in 8f6c
Go back to
Iron binding site 2 out
of 4 in the E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer

Mono view
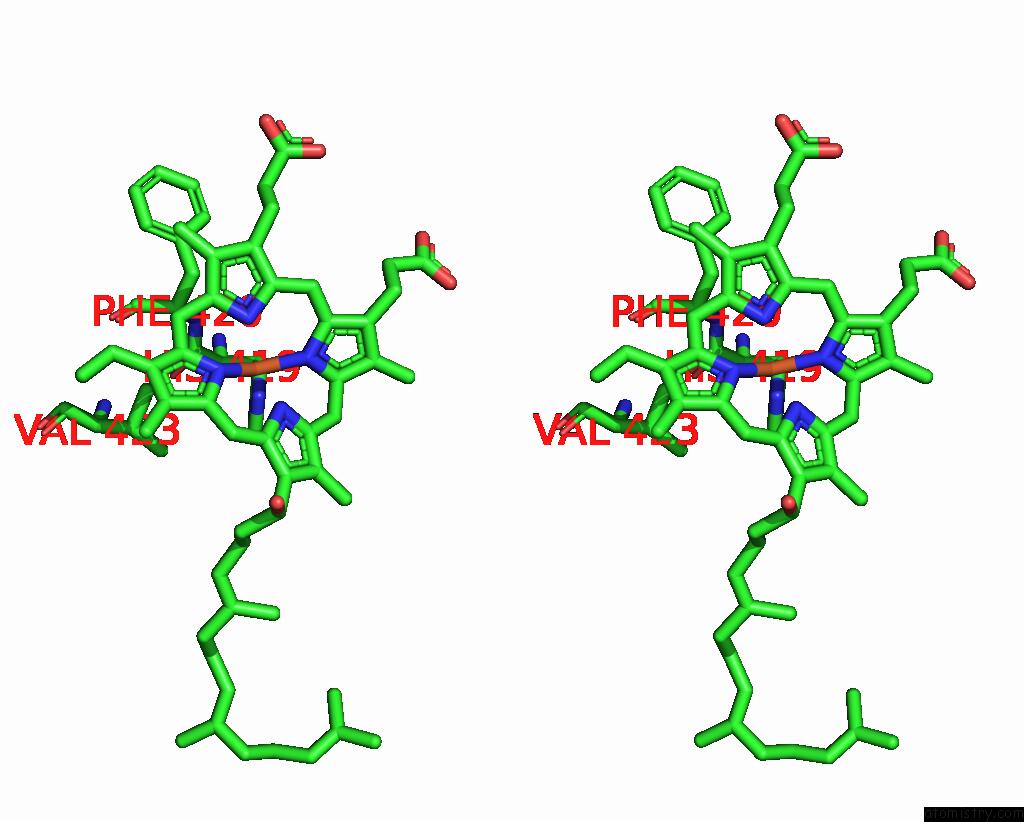
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer within 5.0Å range:
|
Iron binding site 3 out of 4 in 8f6c
Go back to
Iron binding site 3 out
of 4 in the E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer
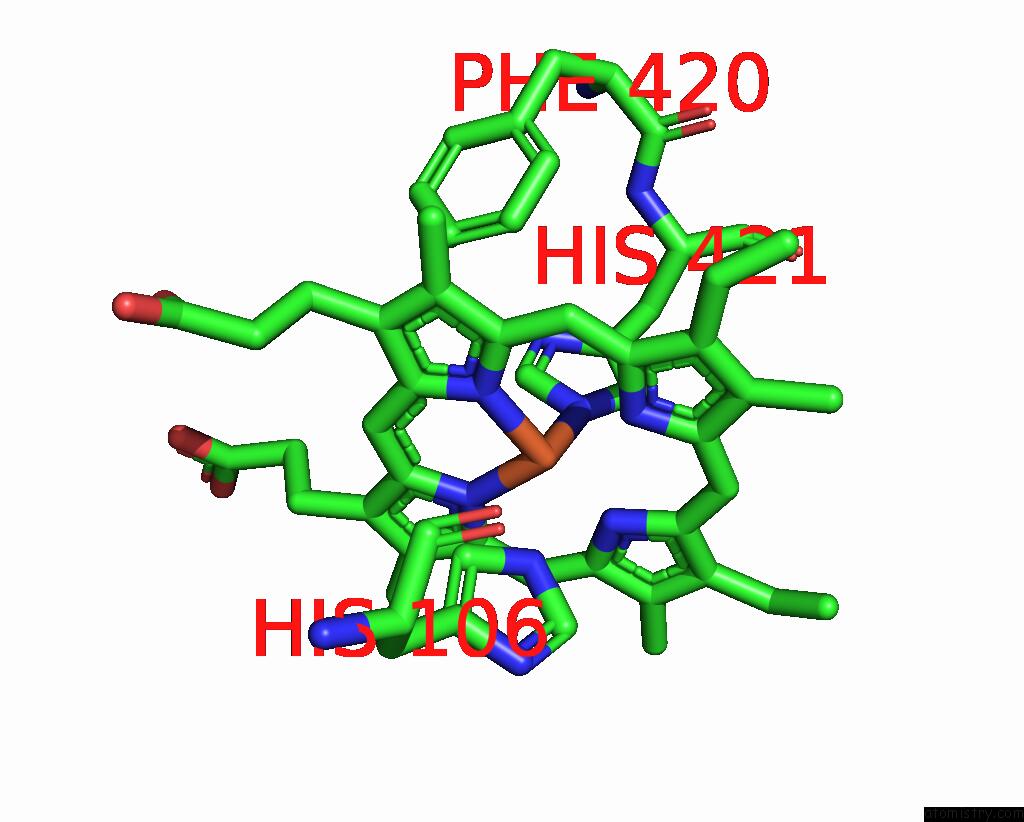
Mono view
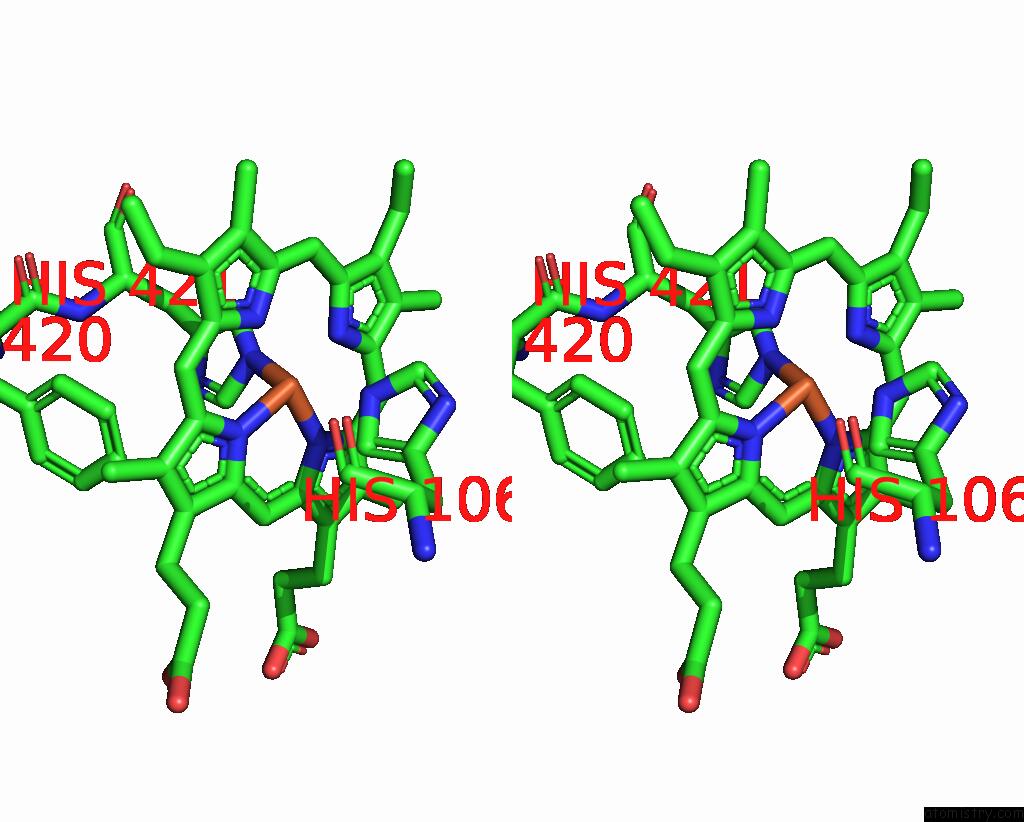
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer within 5.0Å range:
|
Iron binding site 4 out of 4 in 8f6c
Go back to
Iron binding site 4 out
of 4 in the E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer
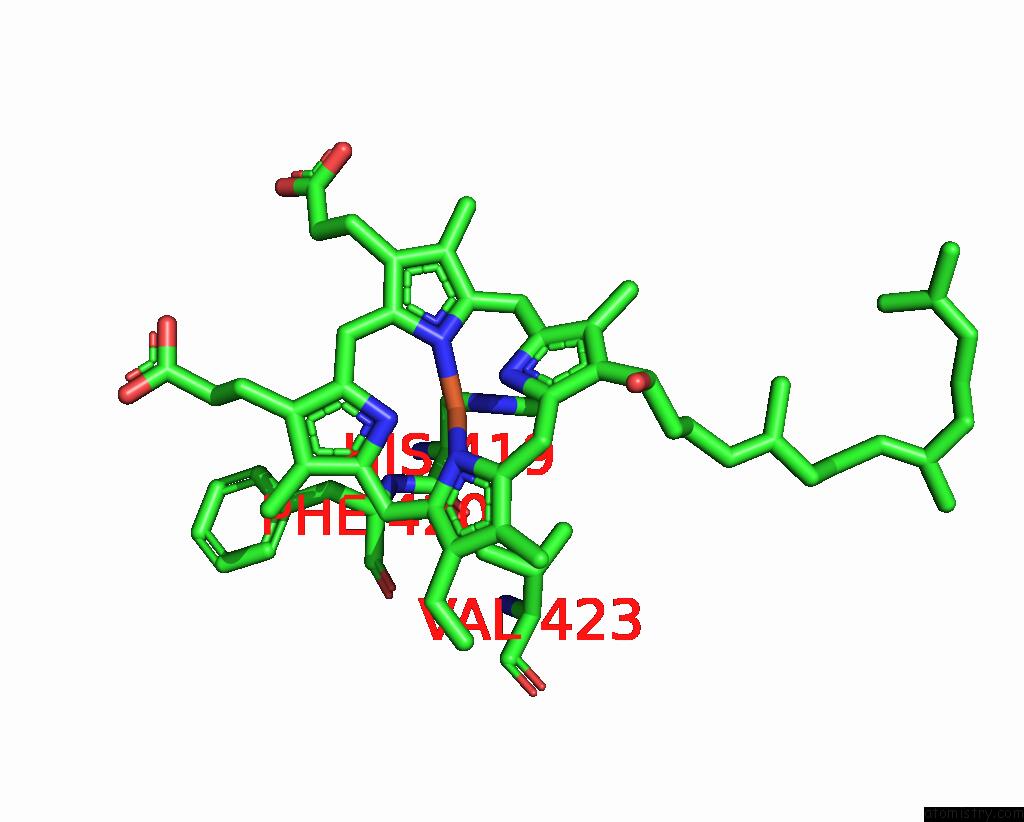
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of E. Coli Cytochrome BO3 Ubiquinol Oxidase Dimer within 5.0Å range:
|
Reference:
Y.Guo,
E.Karimullina,
D.Borek,
A.Savchenko.
Monomer and Dimer Structures of E. Coli Cytochrome BO3 Ubiquinol Oxidase To Be Published.
Page generated: Thu Aug 7 16:50:57 2025
Last articles
Mg in 2IS6Mg in 2ISI
Mg in 2ITN
Mg in 2ISP
Mg in 2ISO
Mg in 2IOU
Mg in 2IS4
Mg in 2IOH
Mg in 2IQQ
Mg in 2IQ1