Iron »
PDB 8euh-8f9n »
8f9i »
Iron in PDB 8f9i: H64A Swmb-Etno Adduct
Protein crystallography data
The structure of H64A Swmb-Etno Adduct, PDB code: 8f9i
was solved by
V.E.Herrera,
L.N.Thomas,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.38 / 1.80 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 41.825, 76.772, 50.529, 90, 103.8, 90 |
| R / Rfree (%) | 17.5 / 22.7 |
Iron Binding Sites:
The binding sites of Iron atom in the H64A Swmb-Etno Adduct
(pdb code 8f9i). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the H64A Swmb-Etno Adduct, PDB code: 8f9i:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the H64A Swmb-Etno Adduct, PDB code: 8f9i:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 8f9i
Go back to
Iron binding site 1 out
of 2 in the H64A Swmb-Etno Adduct
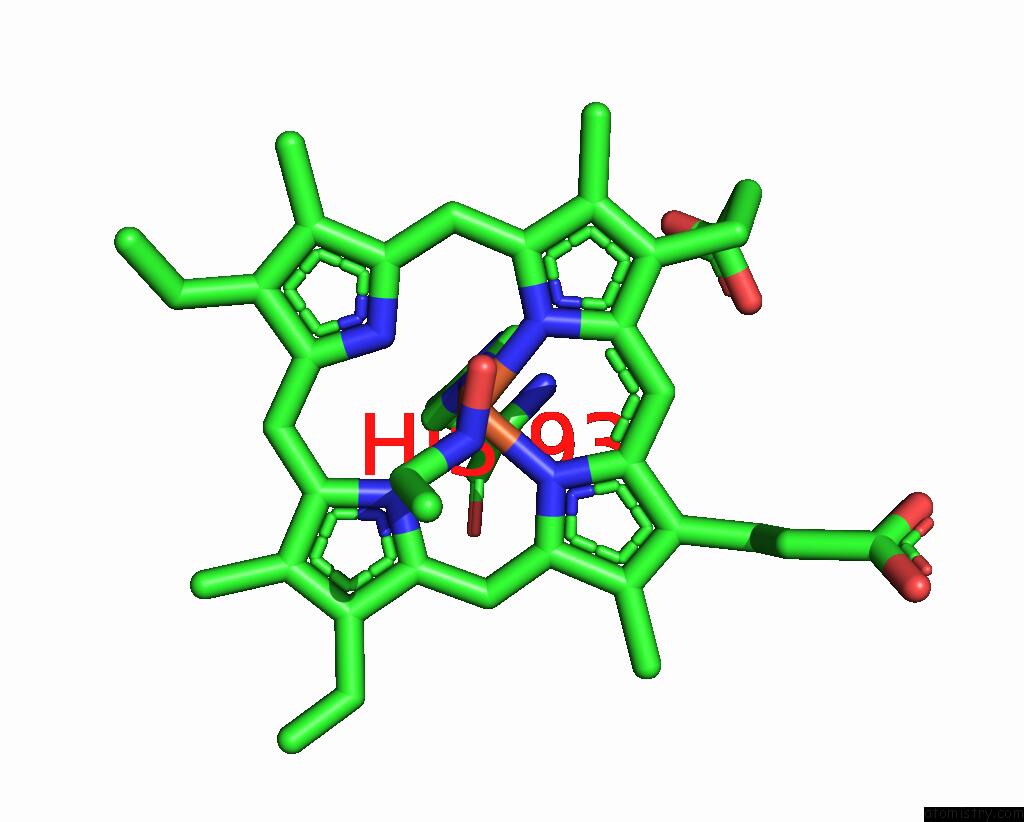
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of H64A Swmb-Etno Adduct within 5.0Å range:
|
Iron binding site 2 out of 2 in 8f9i
Go back to
Iron binding site 2 out
of 2 in the H64A Swmb-Etno Adduct
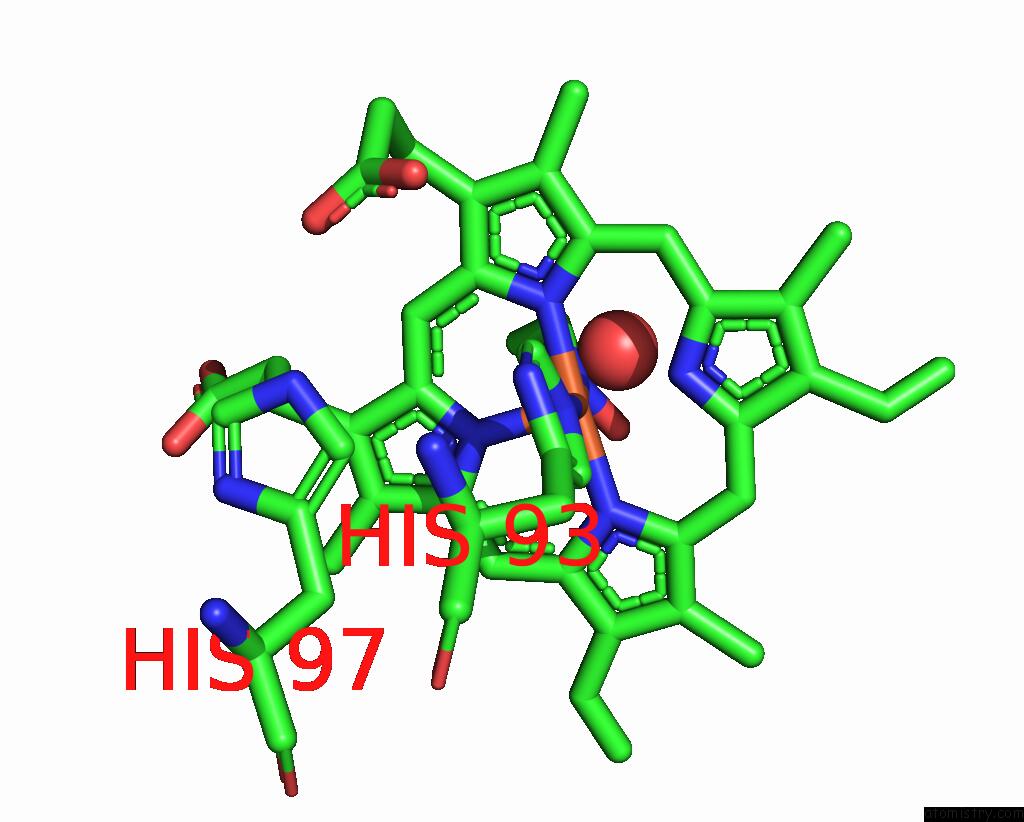
Mono view
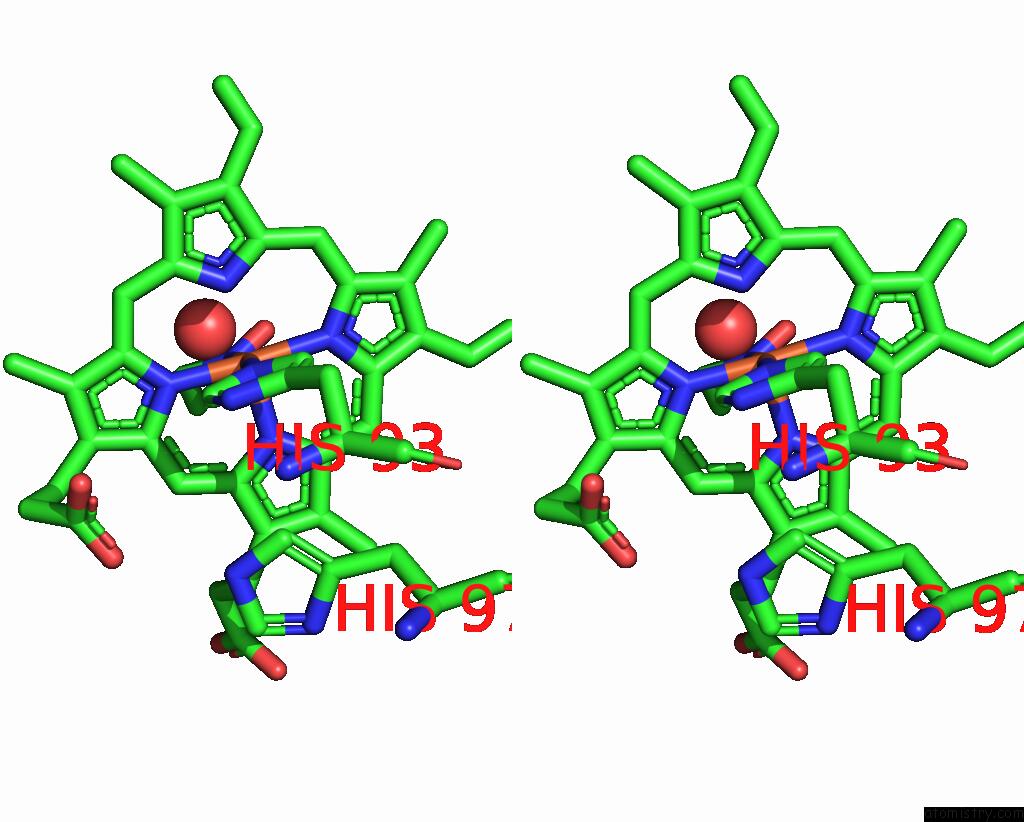
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of H64A Swmb-Etno Adduct within 5.0Å range:
|
Reference:
V.E.Herrera,
T.P.Charles,
T.G.Scott,
K.Y.Prather,
N.T.Nguyen,
C.D.Sohl,
L.M.Thomas,
G.B.Richter-Addo.
Insights Into Nitrosoalkane Binding to Myoglobin Provided By Crystallography of Wild-Type and Distal Pocket Mutant Derivatives. Biochemistry V. 62 1406 2023.
ISSN: ISSN 0006-2960
PubMed: 37011611
DOI: 10.1021/ACS.BIOCHEM.2C00725
Page generated: Thu Aug 7 16:53:22 2025
ISSN: ISSN 0006-2960
PubMed: 37011611
DOI: 10.1021/ACS.BIOCHEM.2C00725
Last articles
Mg in 2IS6Mg in 2ISI
Mg in 2ITN
Mg in 2ISP
Mg in 2ISO
Mg in 2IOU
Mg in 2IS4
Mg in 2IOH
Mg in 2IQQ
Mg in 2IQ1