Iron »
PDB 8gbt-8hbe »
8gy2 »
Iron in PDB 8gy2: Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans
Enzymatic activity of Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans
All present enzymatic activity of Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans:
1.1.5.5;
1.1.5.5;
Other elements in 8gy2:
The structure of Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans also contains other interesting chemical elements:
| Calcium | (Ca) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans
(pdb code 8gy2). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans, PDB code: 8gy2:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans, PDB code: 8gy2:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 8gy2
Go back to
Iron binding site 1 out
of 4 in the Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans within 5.0Å range:
|
Iron binding site 2 out of 4 in 8gy2
Go back to
Iron binding site 2 out
of 4 in the Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans within 5.0Å range:
|
Iron binding site 3 out of 4 in 8gy2
Go back to
Iron binding site 3 out
of 4 in the Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans
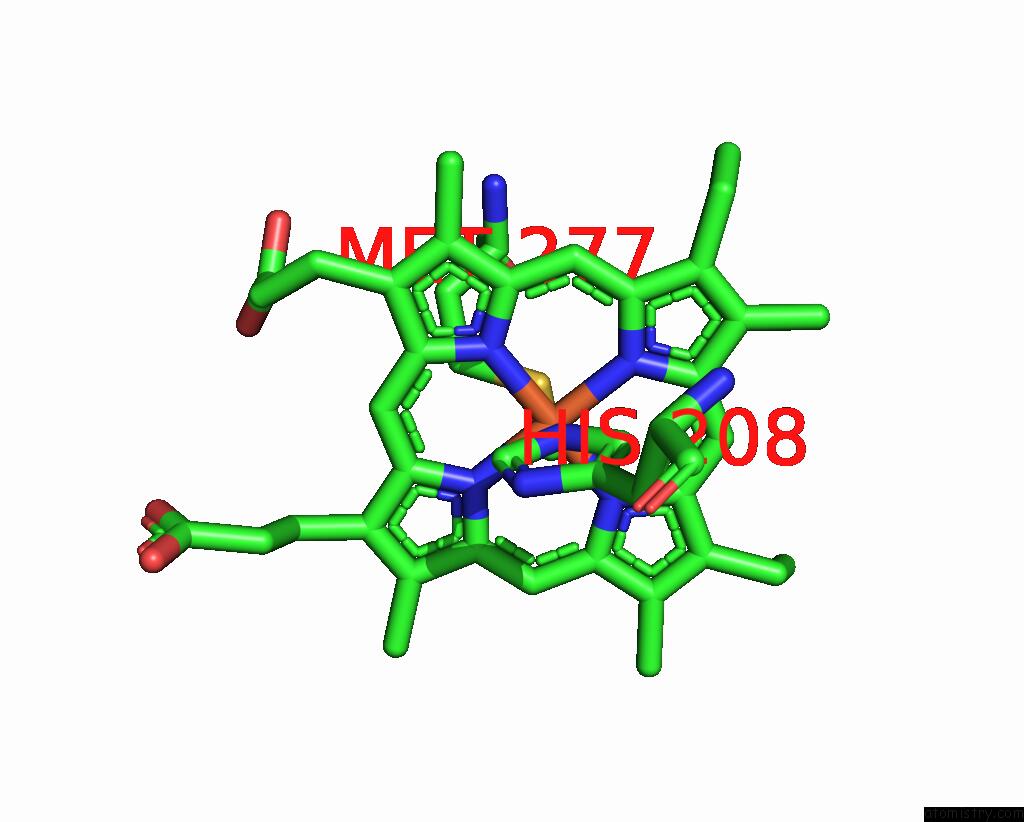
Mono view
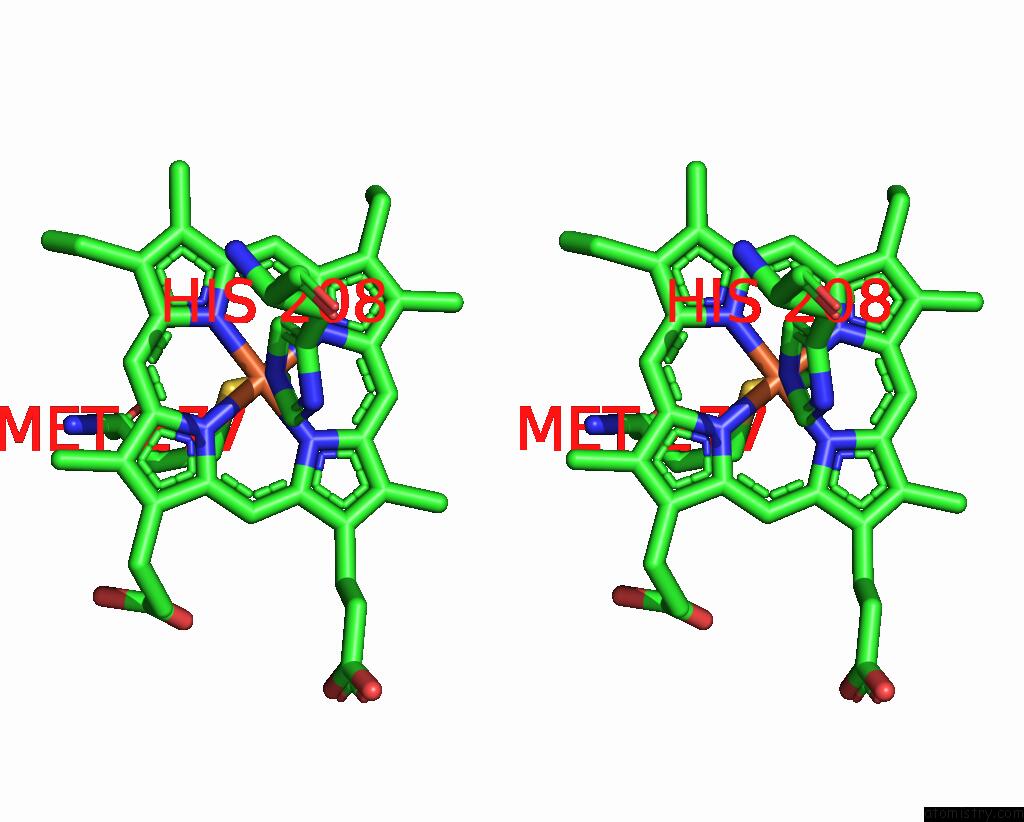
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans within 5.0Å range:
|
Iron binding site 4 out of 4 in 8gy2
Go back to
Iron binding site 4 out
of 4 in the Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans
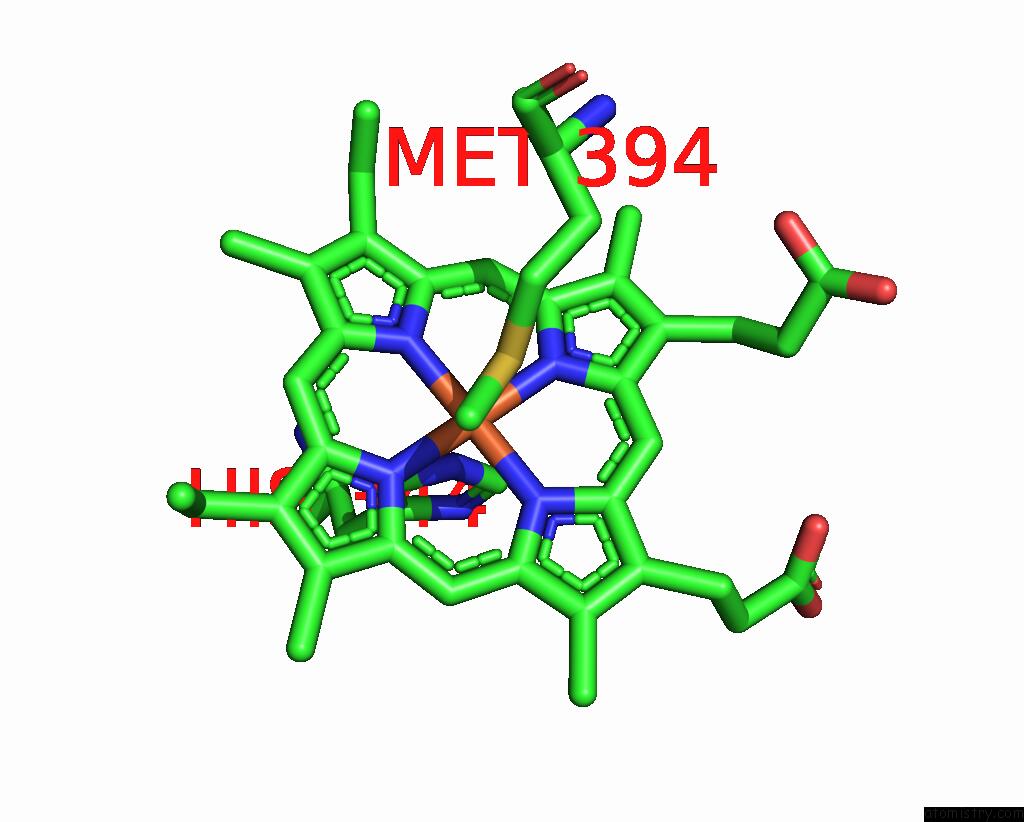
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cryo-Em Structure of Membrane-Bound Alcohol Dehydrogenase From Gluconobacter Oxydans within 5.0Å range:
|
Reference:
T.Adachi,
T.Miyata,
F.Makino,
H.Tanaka,
K.Namba,
K.Kano,
K.Sowa,
Y.Kitazumi,
O.Shirai.
Experimental and Theoretical Insights Into Bienzymatic Cascade For Mediatorless Bioelectrochemical Ethanol Oxidation with Alcohol and Aldehyde Dehydrogenases Acs Catalysis V. 13 7955 2023.
ISSN: ESSN 2155-5435
DOI: 10.1021/ACSCATAL.3C01962
Page generated: Sat Aug 10 05:03:20 2024
ISSN: ESSN 2155-5435
DOI: 10.1021/ACSCATAL.3C01962
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1