Iron »
PDB 9g8f-9hek »
9gcz »
Iron in PDB 9gcz: Xusb Lipoprotein Bound to Ferric Enterobactin
Protein crystallography data
The structure of Xusb Lipoprotein Bound to Ferric Enterobactin, PDB code: 9gcz
was solved by
A.Silale,
H.Mark,
A.Basle,
B.Van Den Berg,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.05 / 1.50 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 59.992, 48.18, 135.466, 90, 96.79, 90 |
| R / Rfree (%) | 20.4 / 24.4 |
Other elements in 9gcz:
The structure of Xusb Lipoprotein Bound to Ferric Enterobactin also contains other interesting chemical elements:
| Sodium | (Na) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Xusb Lipoprotein Bound to Ferric Enterobactin
(pdb code 9gcz). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Xusb Lipoprotein Bound to Ferric Enterobactin, PDB code: 9gcz:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Xusb Lipoprotein Bound to Ferric Enterobactin, PDB code: 9gcz:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 9gcz
Go back to
Iron binding site 1 out
of 2 in the Xusb Lipoprotein Bound to Ferric Enterobactin
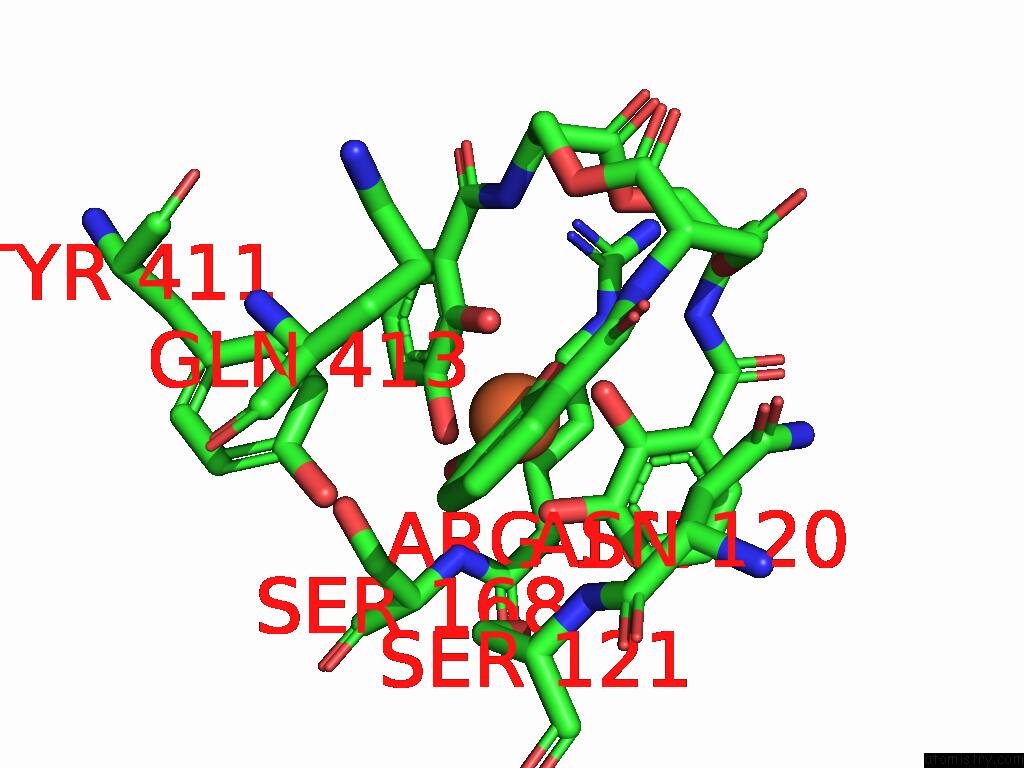
Mono view
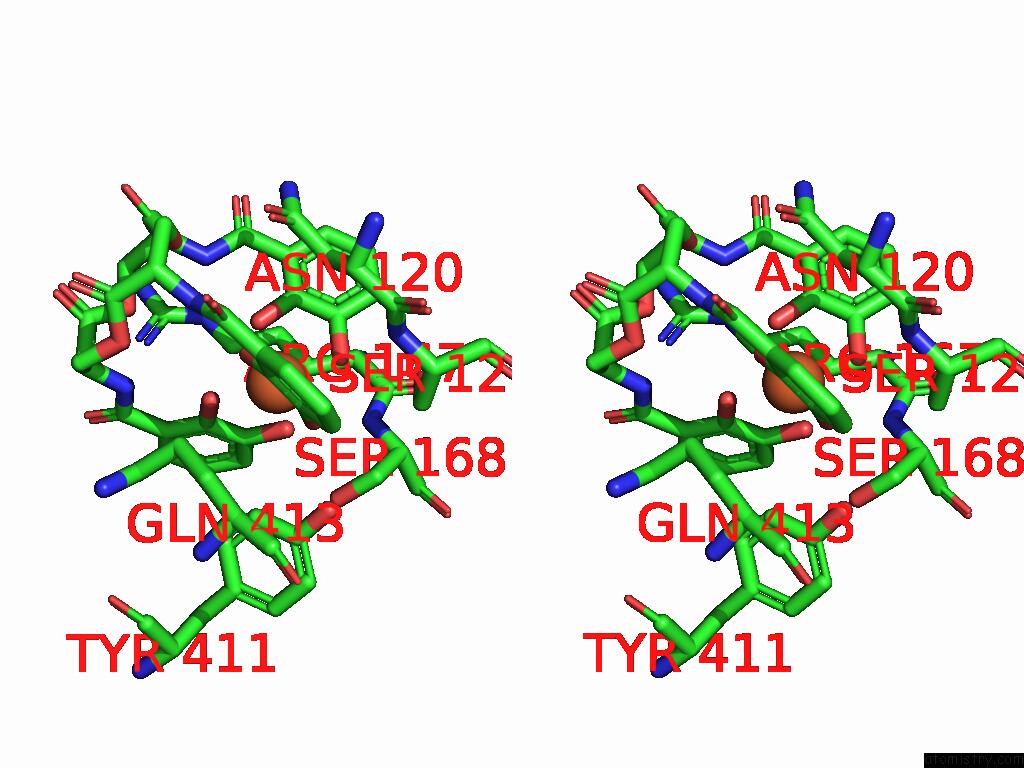
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Xusb Lipoprotein Bound to Ferric Enterobactin within 5.0Å range:
|
Iron binding site 2 out of 2 in 9gcz
Go back to
Iron binding site 2 out
of 2 in the Xusb Lipoprotein Bound to Ferric Enterobactin
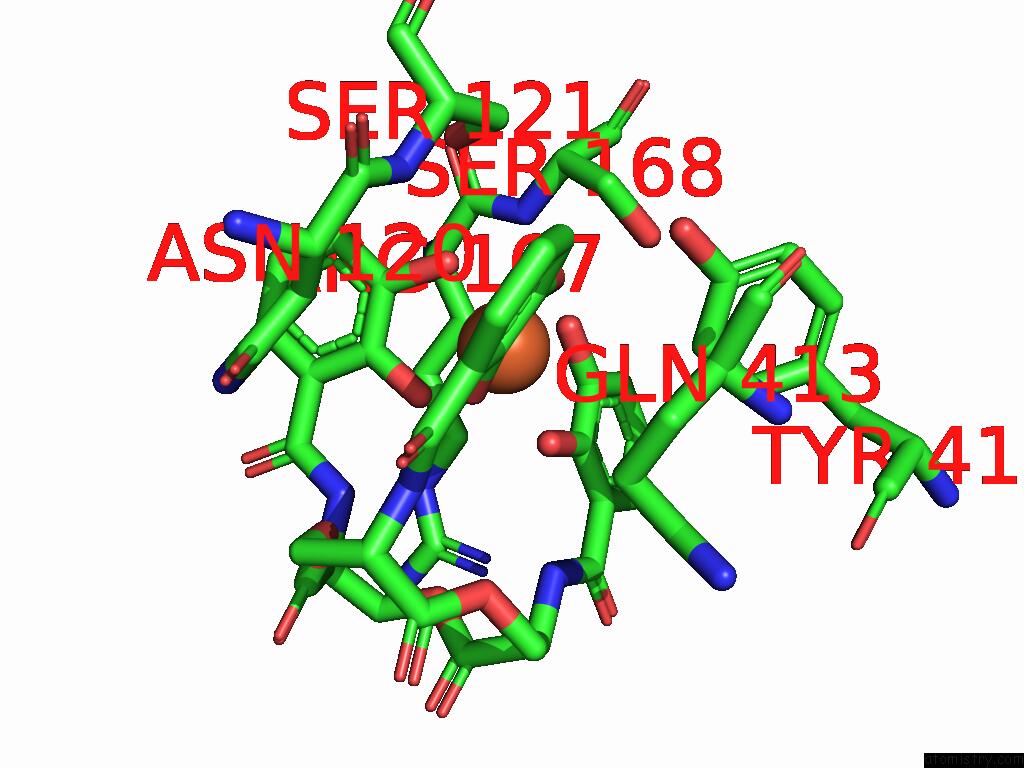
Mono view
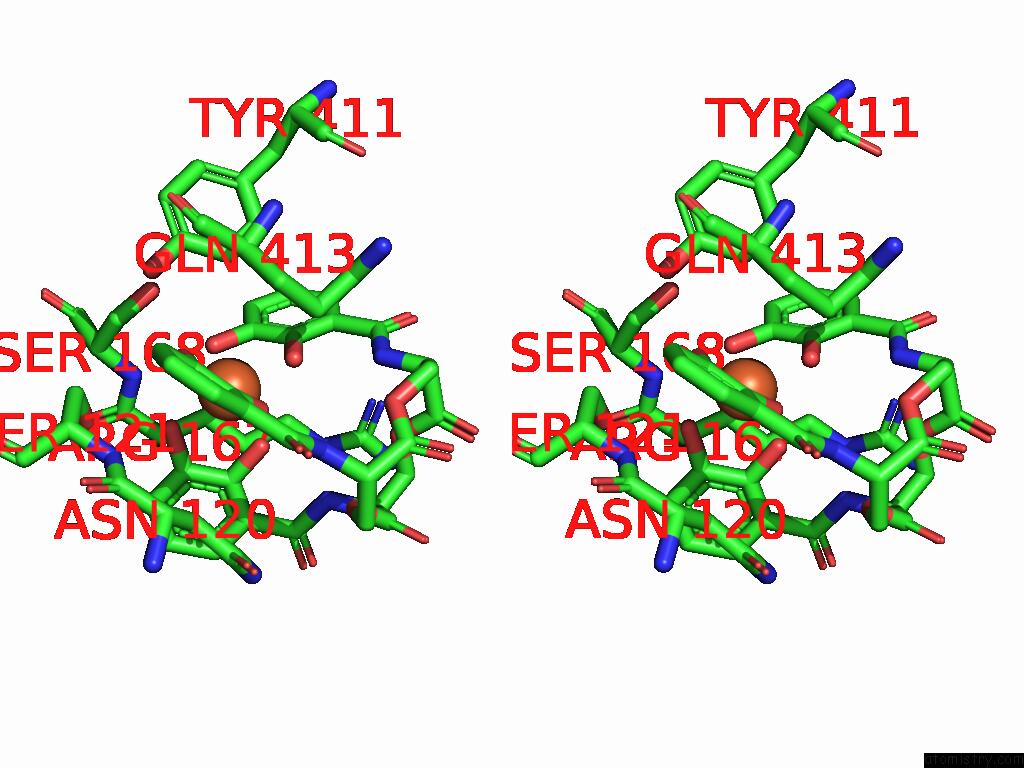
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Xusb Lipoprotein Bound to Ferric Enterobactin within 5.0Å range:
|
Reference:
A.Silale,
Y.L.Soo,
H.Mark,
A.Basle,
B.Van Den Berg.
Structural Basis of Iron Piracy By Human Gut Bacteroides To Be Published.
Page generated: Sat Aug 23 03:13:08 2025
Last articles
Mn in 9LJUMn in 9LJW
Mn in 9LJS
Mn in 9LJR
Mn in 9LJT
Mn in 9LJV
Mg in 9UA2
Mg in 9R96
Mg in 9VM1
Mg in 9P01