Iron »
PDB 9iyl-9k3u »
9jqa »
Iron in PDB 9jqa: Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions
Enzymatic activity of Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions
All present enzymatic activity of Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions:
1.1.99.11;
1.1.99.11;
Iron Binding Sites:
The binding sites of Iron atom in the Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions
(pdb code 9jqa). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 5 binding sites of Iron where determined in the Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions, PDB code: 9jqa:
Jump to Iron binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Iron where determined in the Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions, PDB code: 9jqa:
Jump to Iron binding site number: 1; 2; 3; 4; 5;
Iron binding site 1 out of 5 in 9jqa
Go back to
Iron binding site 1 out
of 5 in the Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions
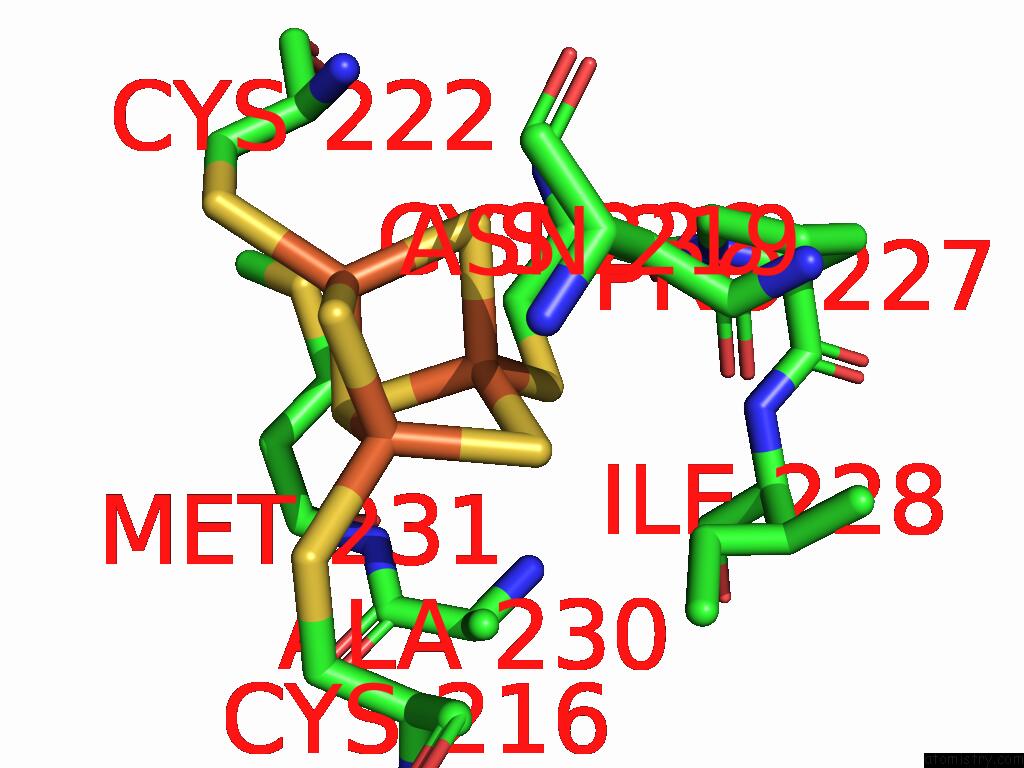
Mono view
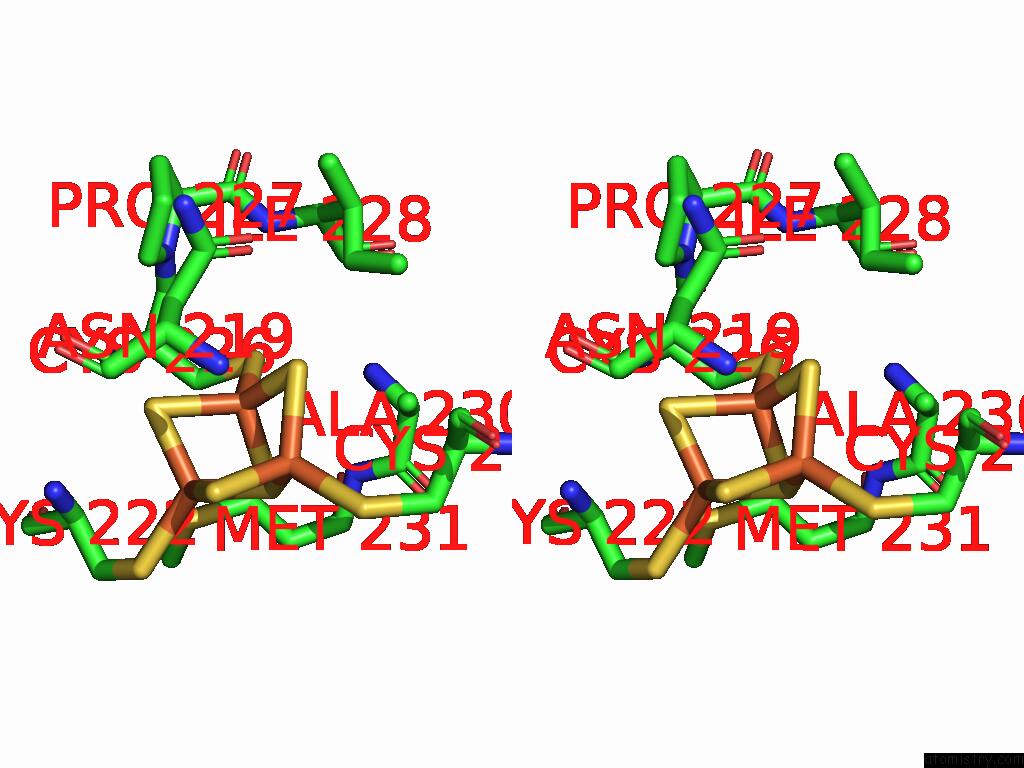
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions within 5.0Å range:
|
Iron binding site 2 out of 5 in 9jqa
Go back to
Iron binding site 2 out
of 5 in the Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions
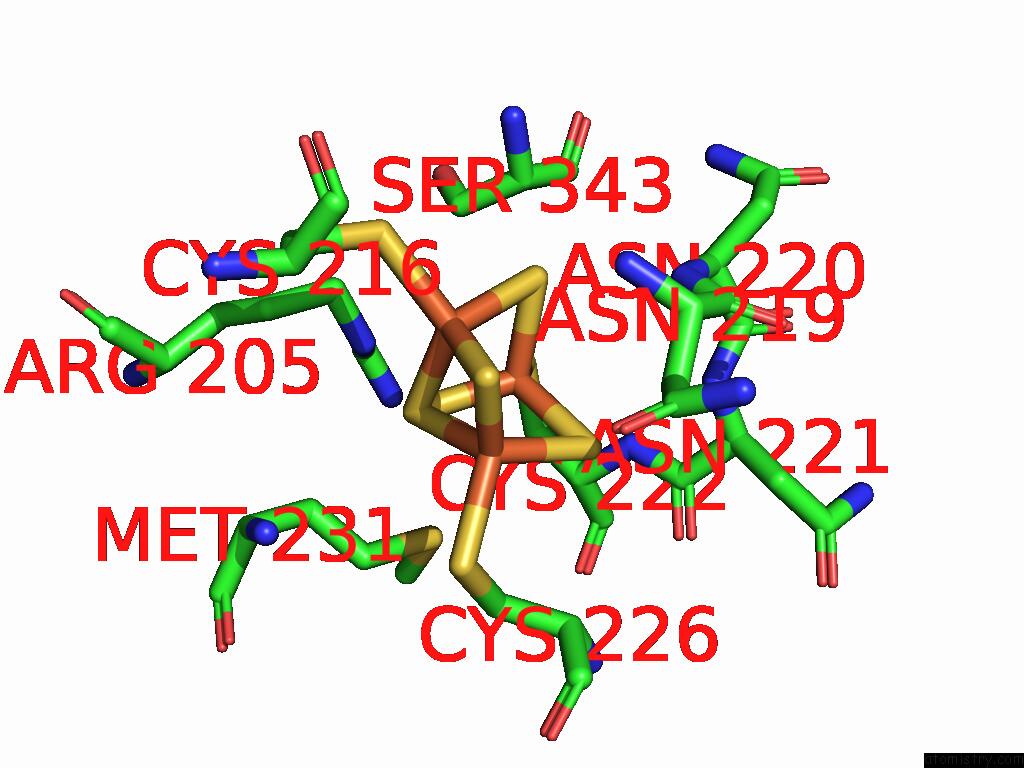
Mono view
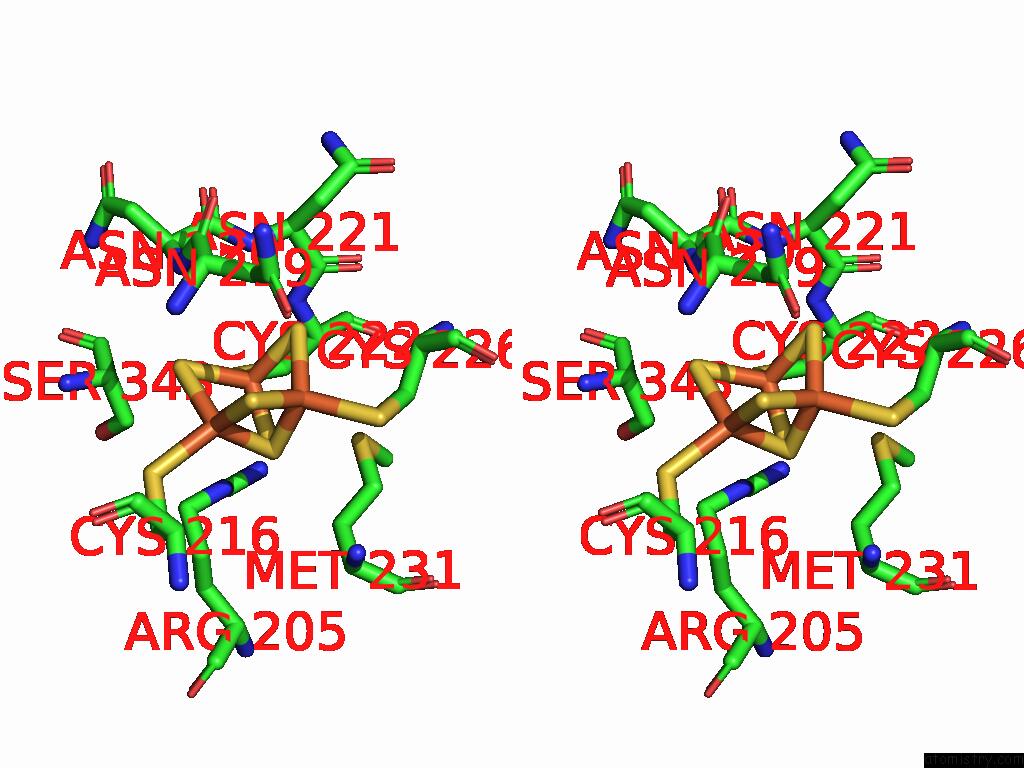
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions within 5.0Å range:
|
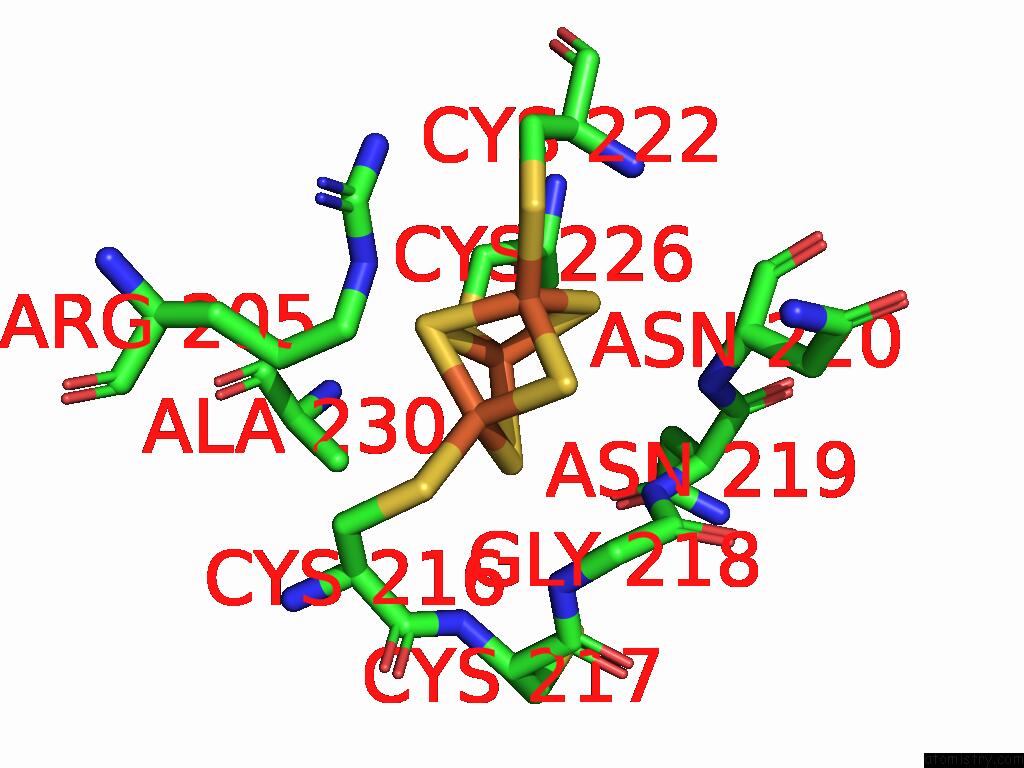
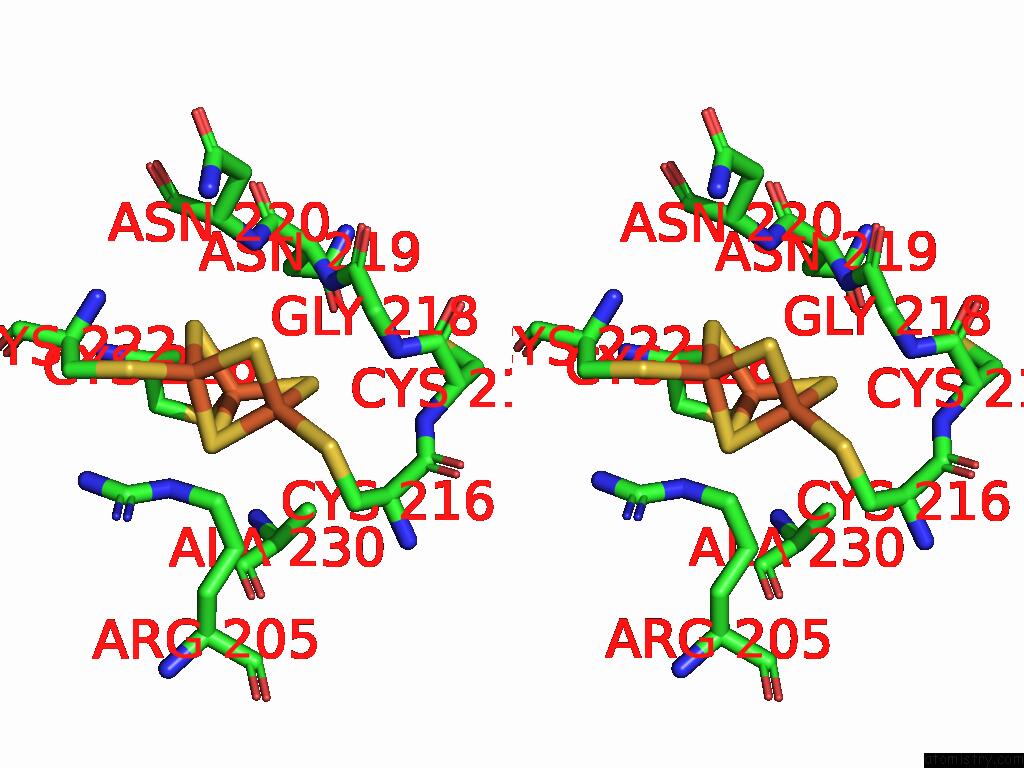
Iron binding site 3 out of 5 in 9jqa
Go back to
Iron binding site 3 out
of 5 in the Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions within 5.0Å range:
|
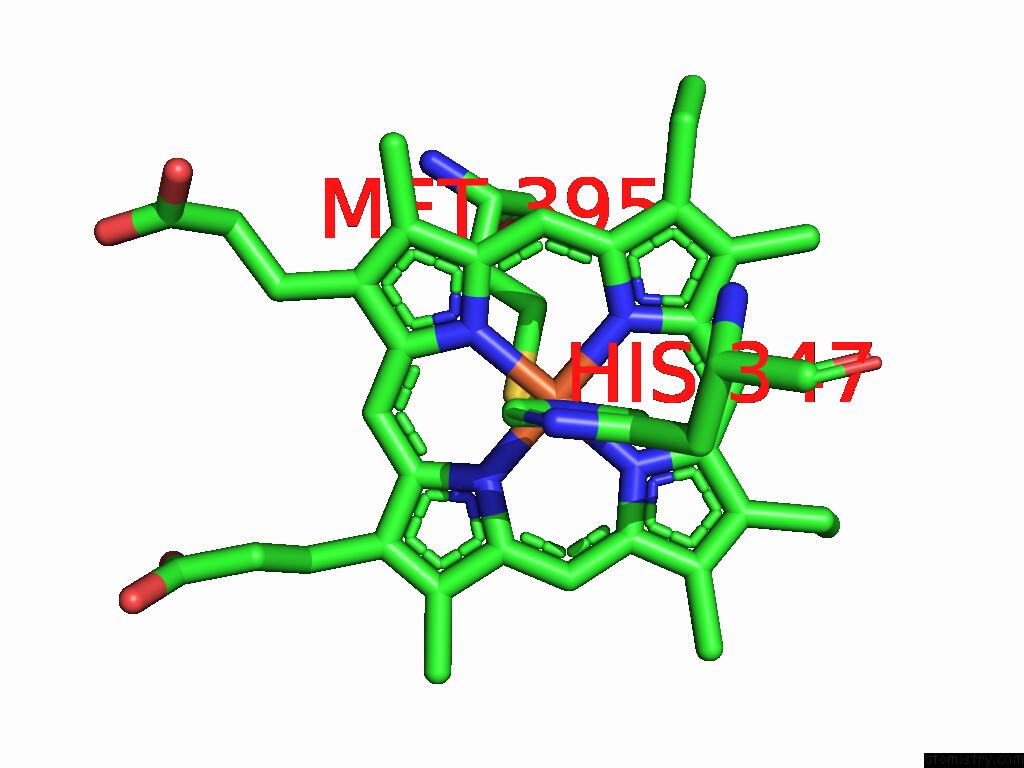
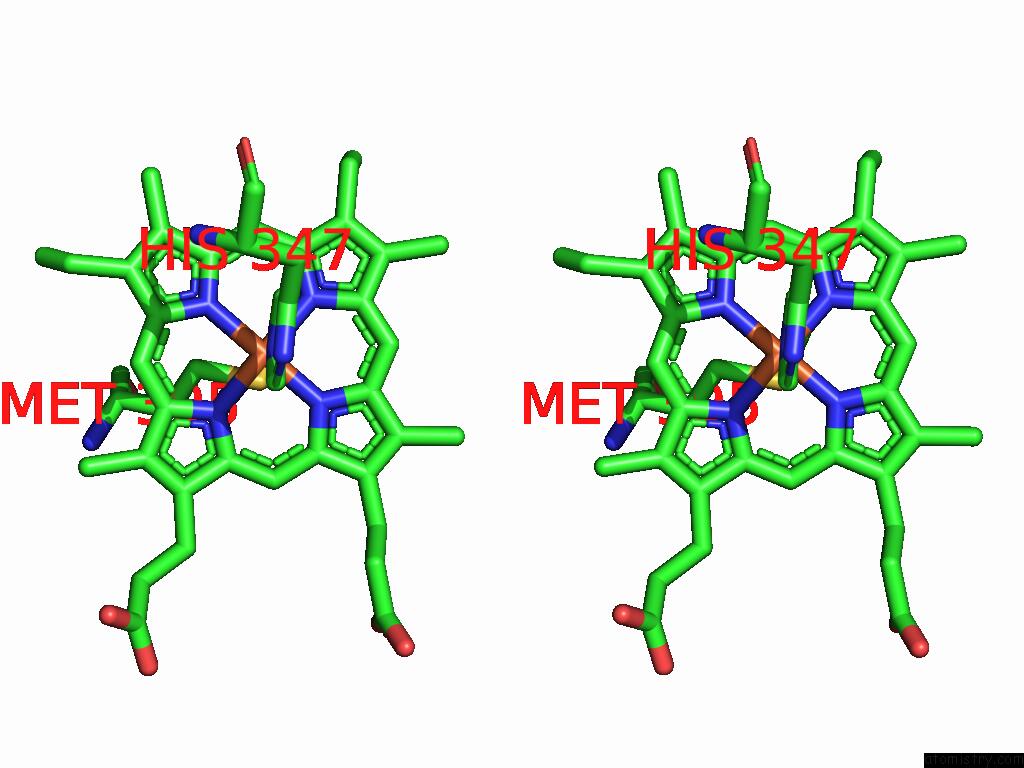
Iron binding site 4 out of 5 in 9jqa
Go back to
Iron binding site 4 out
of 5 in the Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions within 5.0Å range:
|
Iron binding site 5 out of 5 in 9jqa
Go back to
Iron binding site 5 out
of 5 in the Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions
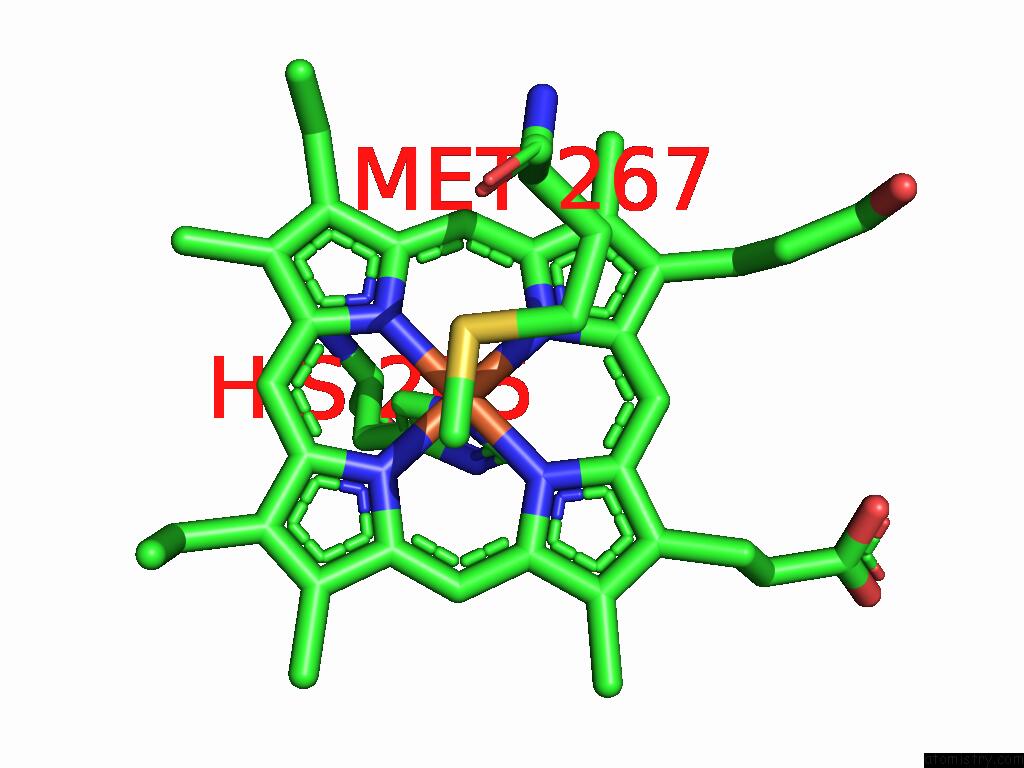
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Cryo-Em Structure of Fructose Dehydrogenase Variant From Gluconobacter Japonicus Truncating Heme 1C and C-Terminal Hydrophobic Regions within 5.0Å range:
|
Reference:
T.Adachi,
K.Ichikawa,
T.Miyata,
F.Makino,
H.Tanaka,
K.Namba,
K.Sowa.
Improved Direct Bioelectrochemical Fructose Oxidation with Surfactant-Free Heterotrimeric Fructose Dehydrogenase Variant Truncating Heme 1C and C-Terminal Hydrophobic Regions Acs Electrochem 2025.
DOI: 10.1021/ACSELECTROCHEM.5C00106
Page generated: Sat Aug 23 03:27:58 2025
DOI: 10.1021/ACSELECTROCHEM.5C00106
Last articles
Mn in 9LJUMn in 9LJW
Mn in 9LJS
Mn in 9LJR
Mn in 9LJT
Mn in 9LJV
Mg in 9UA2
Mg in 9R96
Mg in 9VM1
Mg in 9P01