Iron »
PDB 1b2o-1biy »
1b9r »
Iron in PDB 1b9r: Terpredoxin From Pseudomonas Sp.
Iron Binding Sites:
The binding sites of Iron atom in the Terpredoxin From Pseudomonas Sp.
(pdb code 1b9r). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Terpredoxin From Pseudomonas Sp., PDB code: 1b9r:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Terpredoxin From Pseudomonas Sp., PDB code: 1b9r:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 1b9r
Go back to
Iron binding site 1 out
of 2 in the Terpredoxin From Pseudomonas Sp.
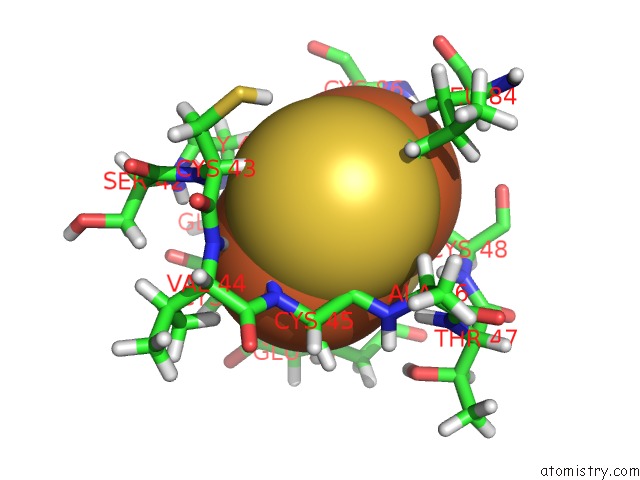
Mono view
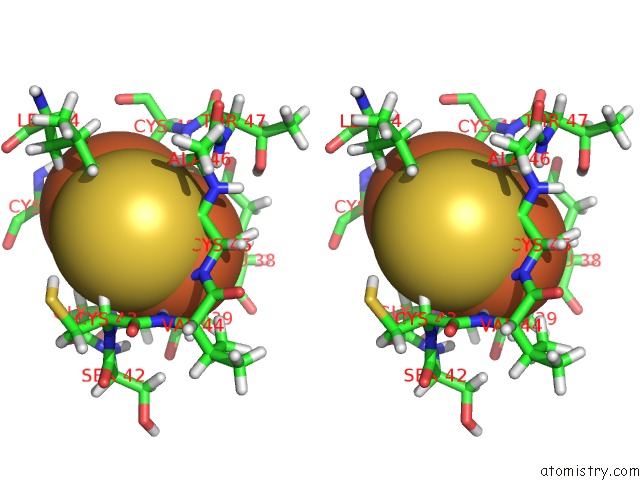
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Terpredoxin From Pseudomonas Sp. within 5.0Å range:
|
Iron binding site 2 out of 2 in 1b9r
Go back to
Iron binding site 2 out
of 2 in the Terpredoxin From Pseudomonas Sp.
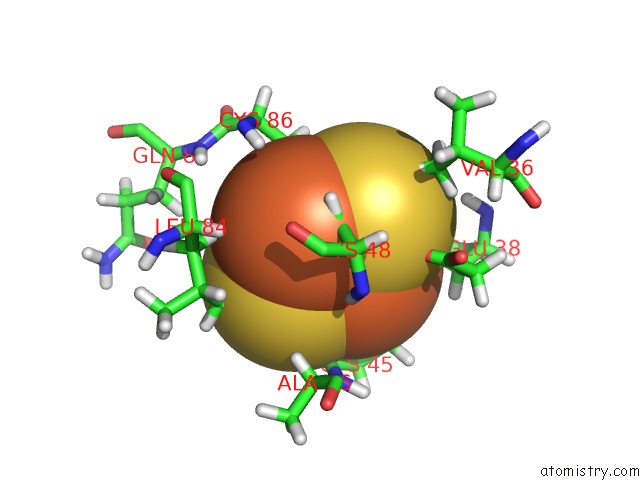
Mono view
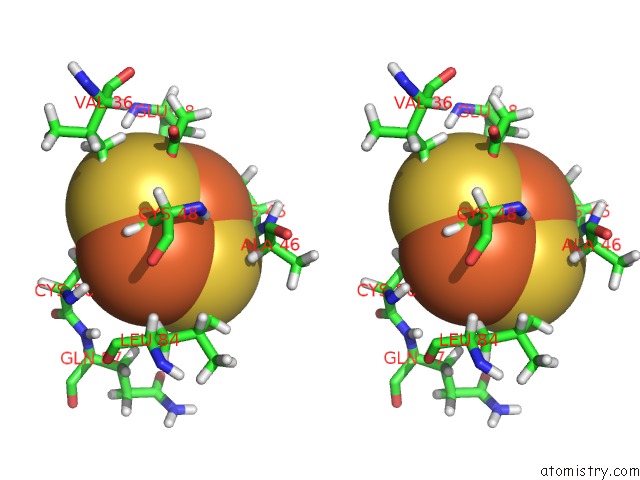
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Terpredoxin From Pseudomonas Sp. within 5.0Å range:
|
Reference:
H.Mo,
S.S.Pochapsky,
T.C.Pochapsky.
A Model For the Solution Structure of Oxidized Terpredoxin, A FE2S2 Ferredoxin From Pseudomonas. Biochemistry V. 38 5666 1999.
ISSN: ISSN 0006-2960
PubMed: 10220356
DOI: 10.1021/BI983063R
Page generated: Wed Jul 16 12:30:14 2025
ISSN: ISSN 0006-2960
PubMed: 10220356
DOI: 10.1021/BI983063R
Last articles
Fe in 2GL3Fe in 2GKN
Fe in 2GKM
Fe in 2GEP
Fe in 2GIW
Fe in 2GJM
Fe in 2GHK
Fe in 2GHH
Fe in 2GHD
Fe in 2GHE