Iron »
PDB 3pcq-3puq »
3pel »
Iron in PDB 3pel: Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity
Protein crystallography data
The structure of Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity, PDB code: 3pel
was solved by
V.S.Bhatt,
S.Zaldivar-Lopez,
D.R.Harris,
C.G.Couto,
P.G.Wang,
A.F.Palmer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 33.50 / 1.90 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 87.973, 88.045, 53.073, 90.00, 103.37, 90.00 |
| R / Rfree (%) | 18.8 / 23.2 |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity
(pdb code 3pel). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity, PDB code: 3pel:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity, PDB code: 3pel:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 3pel
Go back to
Iron binding site 1 out
of 2 in the Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity
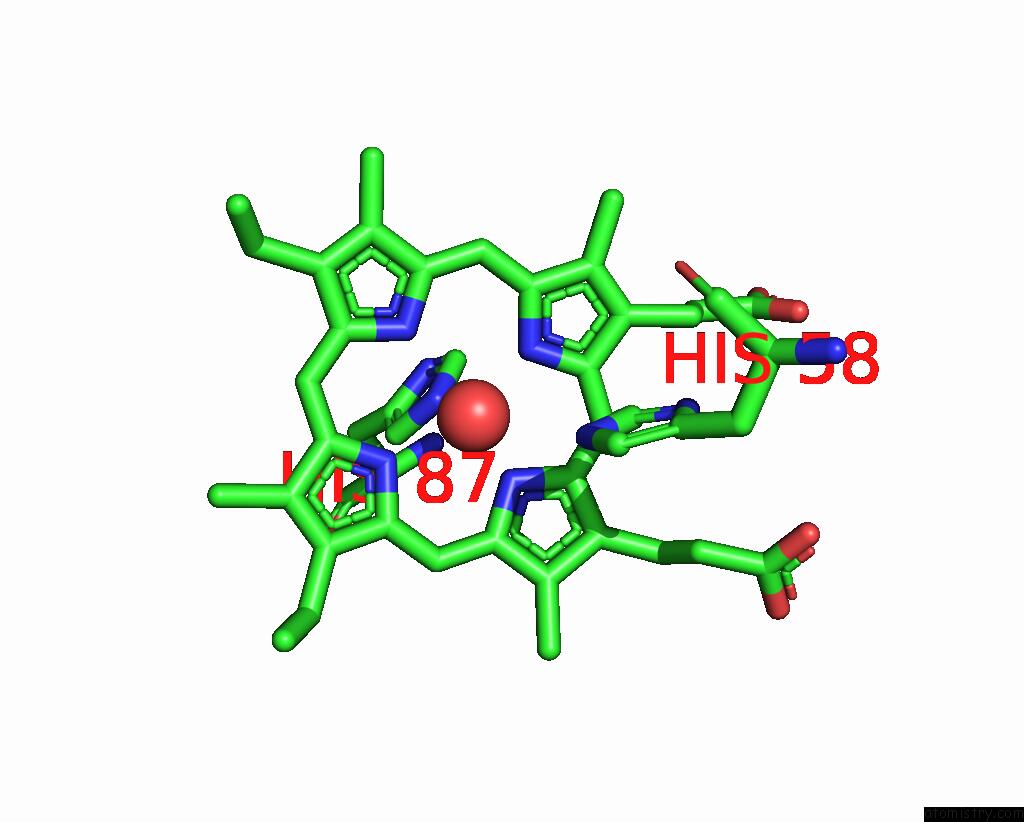
Mono view
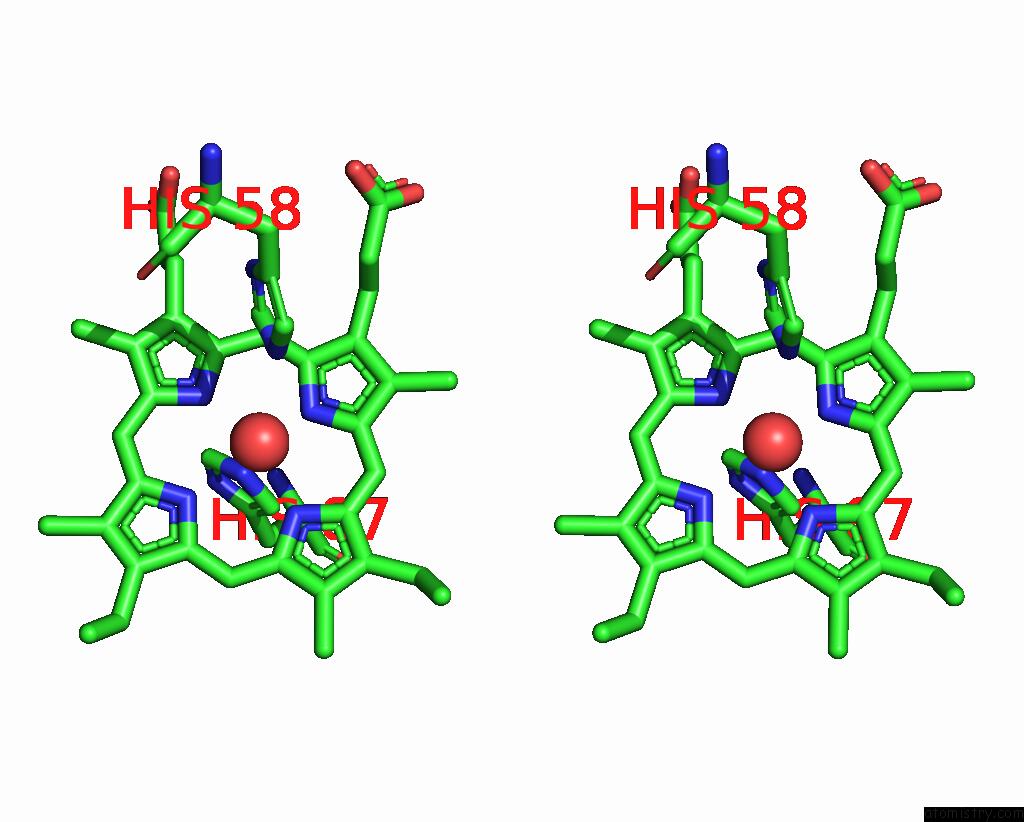
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity within 5.0Å range:
|
Iron binding site 2 out of 2 in 3pel
Go back to
Iron binding site 2 out
of 2 in the Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity
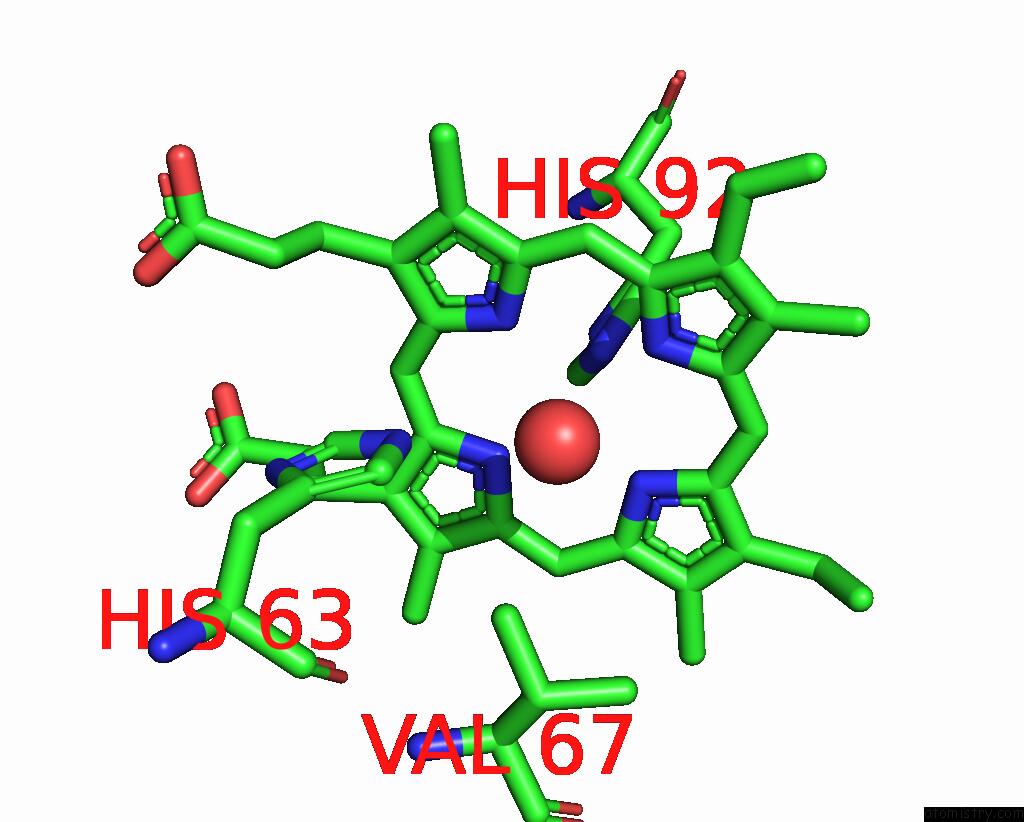
Mono view
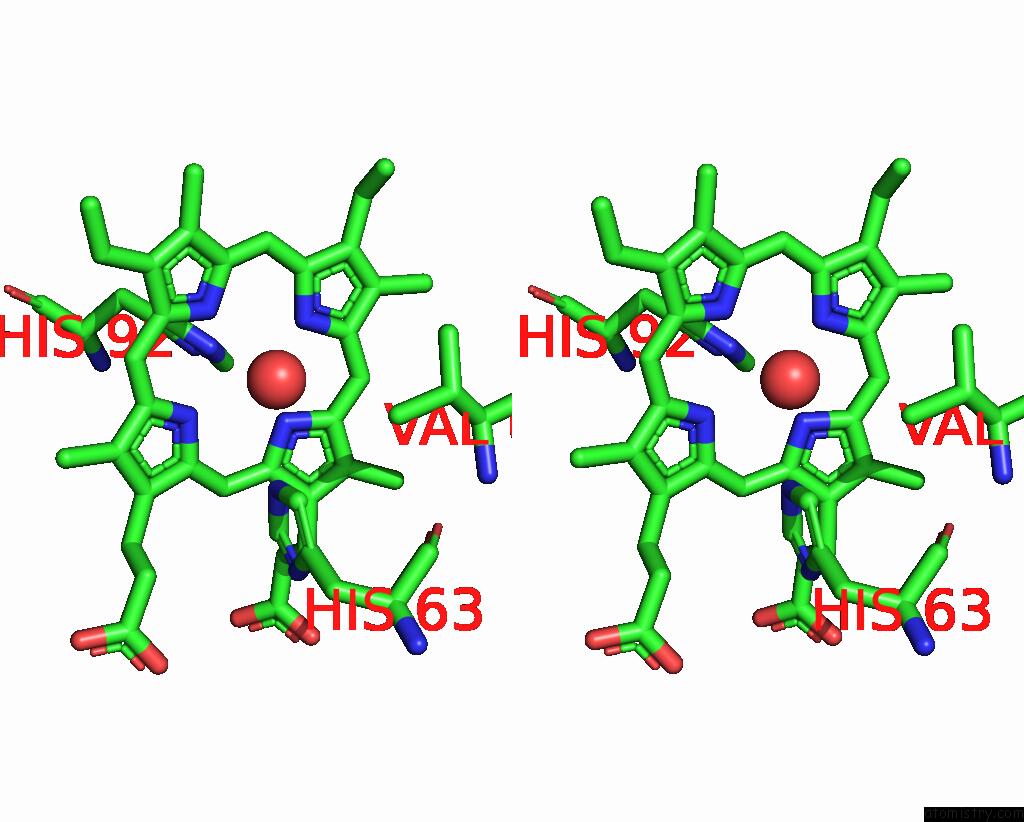
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity within 5.0Å range:
|
Reference:
V.S.Bhatt,
S.Zaldivar-Lopez,
D.R.Harris,
C.G.Couto,
P.G.Wang,
A.F.Palmer.
Structure of Greyhound Hemoglobin: Origin of High Oxygen Affinity. Acta Crystallogr.,Sect.D V. 67 395 2011.
ISSN: ISSN 0907-4449
PubMed: 21543841
DOI: 10.1107/S0907444911006044
Page generated: Tue Aug 5 05:41:14 2025
ISSN: ISSN 0907-4449
PubMed: 21543841
DOI: 10.1107/S0907444911006044
Last articles
Fe in 3WFBFe in 3WCW
Fe in 3WCV
Fe in 3WEC
Fe in 3WCU
Fe in 3WCT
Fe in 3WCP
Fe in 3WCQ
Fe in 3WC8
Fe in 3WAH