Iron »
PDB 5g6f-5h8y »
5gux »
Iron in PDB 5gux: Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon
Enzymatic activity of Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon
All present enzymatic activity of Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon:
1.7.2.5;
1.7.2.5;
Protein crystallography data
The structure of Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon, PDB code: 5gux
was solved by
S.Ishii,
E.Terasaka,
H.Sugimoto,
Y.Shiro,
T.Tosha,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.39 / 3.30 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 89.843, 105.379, 192.577, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.9 / 26.8 |
Other elements in 5gux:
The structure of Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon also contains other interesting chemical elements:
| Xenon | (Xe) | 7 atoms |
| Calcium | (Ca) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon
(pdb code 5gux). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon, PDB code: 5gux:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon, PDB code: 5gux:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 5gux
Go back to
Iron binding site 1 out
of 4 in the Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon
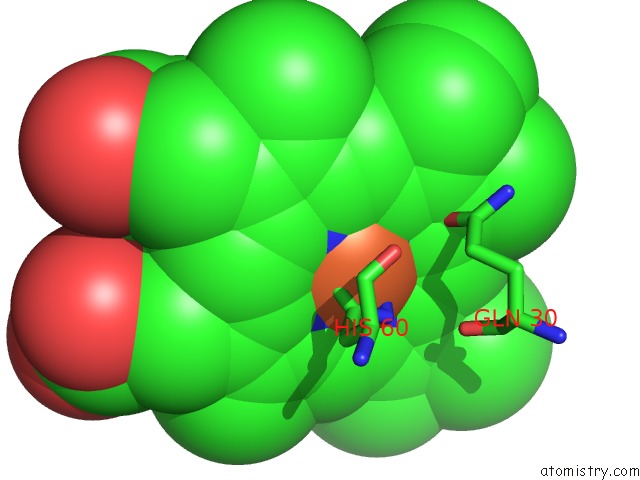
Mono view
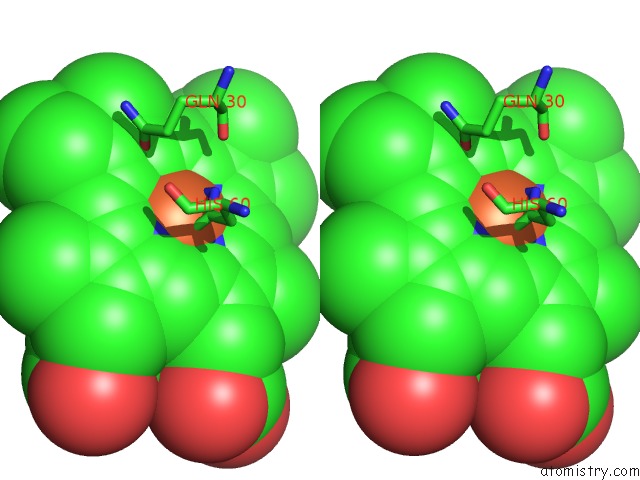
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon within 5.0Å range:
|
Iron binding site 2 out of 4 in 5gux
Go back to
Iron binding site 2 out
of 4 in the Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon
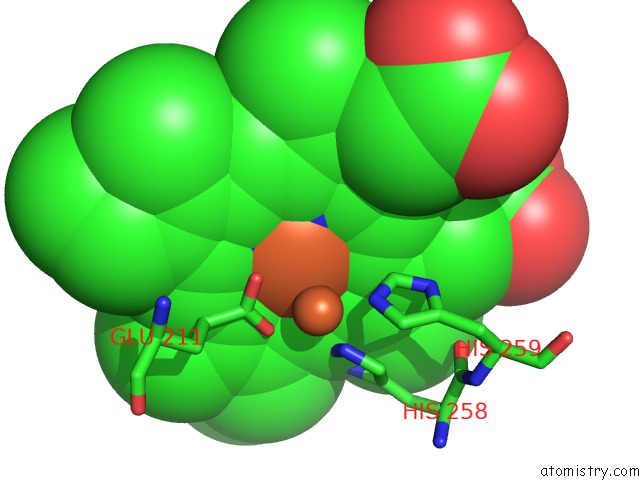
Mono view
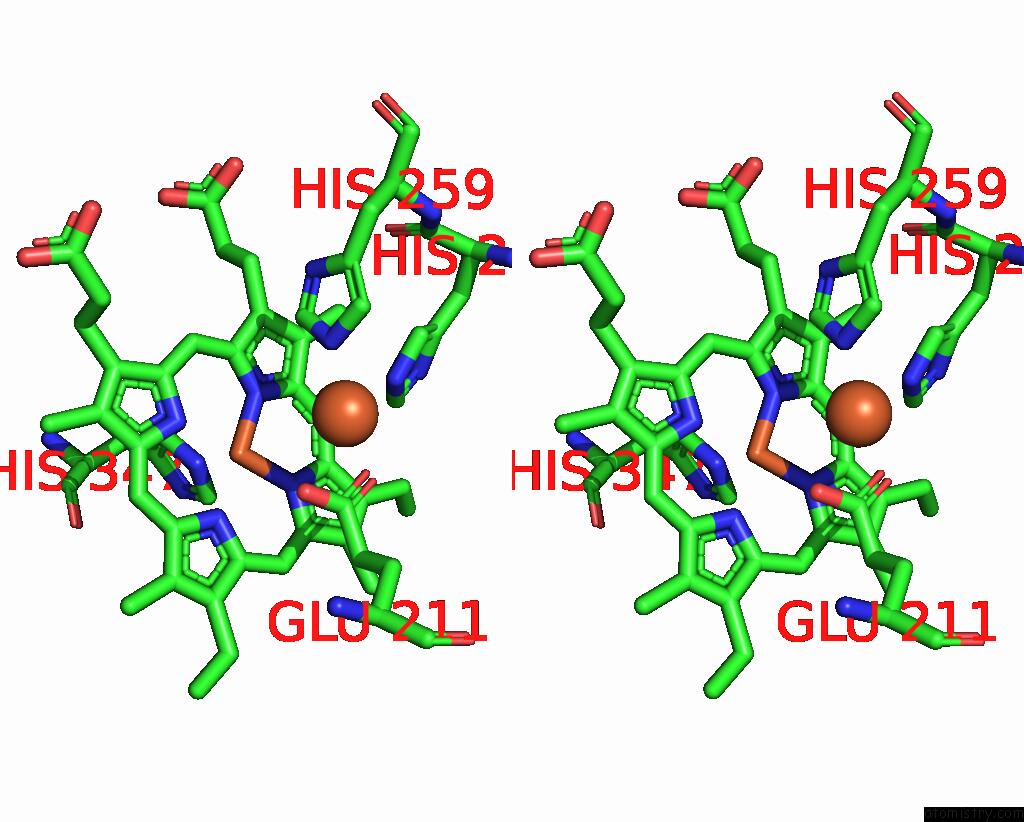
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon within 5.0Å range:
|
Iron binding site 3 out of 4 in 5gux
Go back to
Iron binding site 3 out
of 4 in the Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon
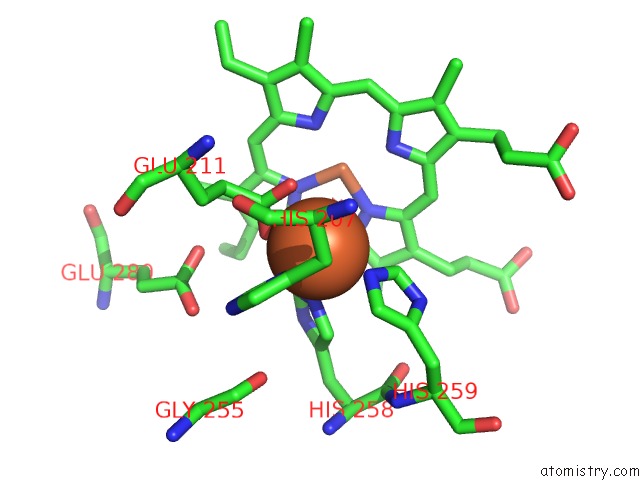
Mono view
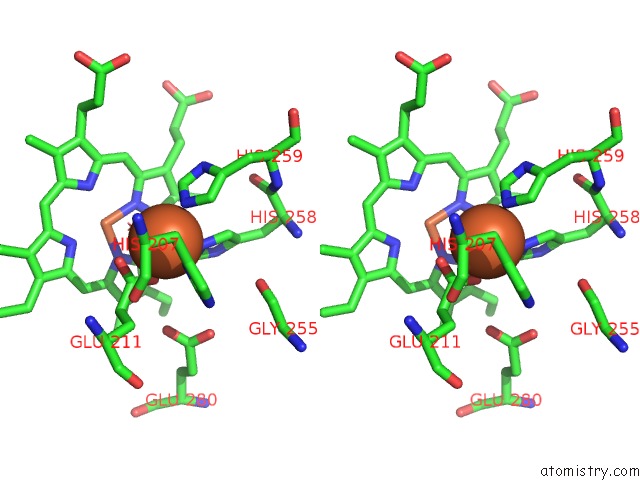
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon within 5.0Å range:
|
Iron binding site 4 out of 4 in 5gux
Go back to
Iron binding site 4 out
of 4 in the Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon
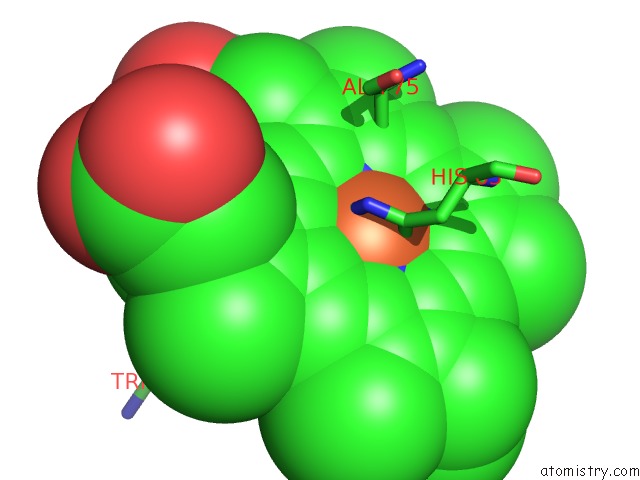
Mono view
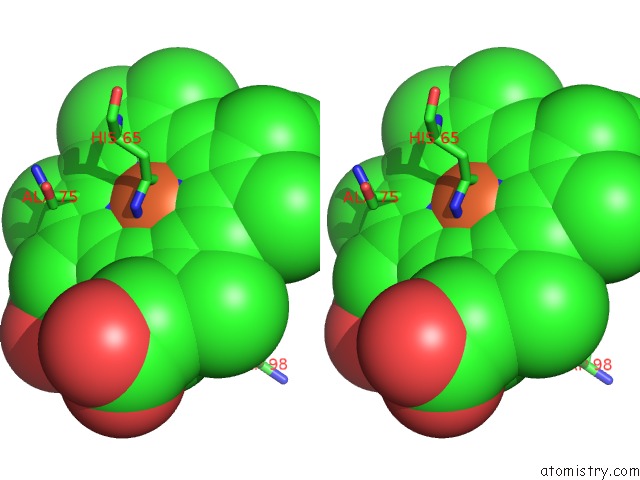
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cytochrome C-Dependent Nitric Oxide Reductase (Cnor) From Pseudomonas Aeruginosa in Complex with Xenon within 5.0Å range:
|
Reference:
E.Terasaka,
K.Yamada,
P.H.Wang,
K.Hosokawa,
R.Yamagiwa,
K.Matsumoto,
S.Ishii,
T.Mori,
K.Yagi,
H.Sawai,
H.Arai,
H.Sugimoto,
Y.Sugita,
Y.Shiro,
T.Tosha.
Dynamics of Nitric Oxide Controlled By Protein Complex in Bacterial System. Proc. Natl. Acad. Sci. V. 114 9888 2017U.S.A..
ISSN: ESSN 1091-6490
PubMed: 28847930
DOI: 10.1073/PNAS.1621301114
Page generated: Tue Aug 6 01:37:47 2024
ISSN: ESSN 1091-6490
PubMed: 28847930
DOI: 10.1073/PNAS.1621301114
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO