Iron »
PDB 5o17-5ok4 »
5ok4 »
Iron in PDB 5ok4: Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2.
Enzymatic activity of Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2.
All present enzymatic activity of Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2.:
1.12.98.2;
1.12.98.2;
Protein crystallography data
The structure of Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2., PDB code: 5ok4
was solved by
T.Wagner,
G.Huang,
E.Bill,
U.Ermler,
K.Ataka,
S.Shima,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 43.47 / 1.29 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 127.418, 127.418, 141.211, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 12.7 / 14.7 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2.
(pdb code 5ok4). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 3 binding sites of Iron where determined in the Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2., PDB code: 5ok4:
Jump to Iron binding site number: 1; 2; 3;
In total 3 binding sites of Iron where determined in the Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2., PDB code: 5ok4:
Jump to Iron binding site number: 1; 2; 3;
Iron binding site 1 out of 3 in 5ok4
Go back to
Iron binding site 1 out
of 3 in the Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2.
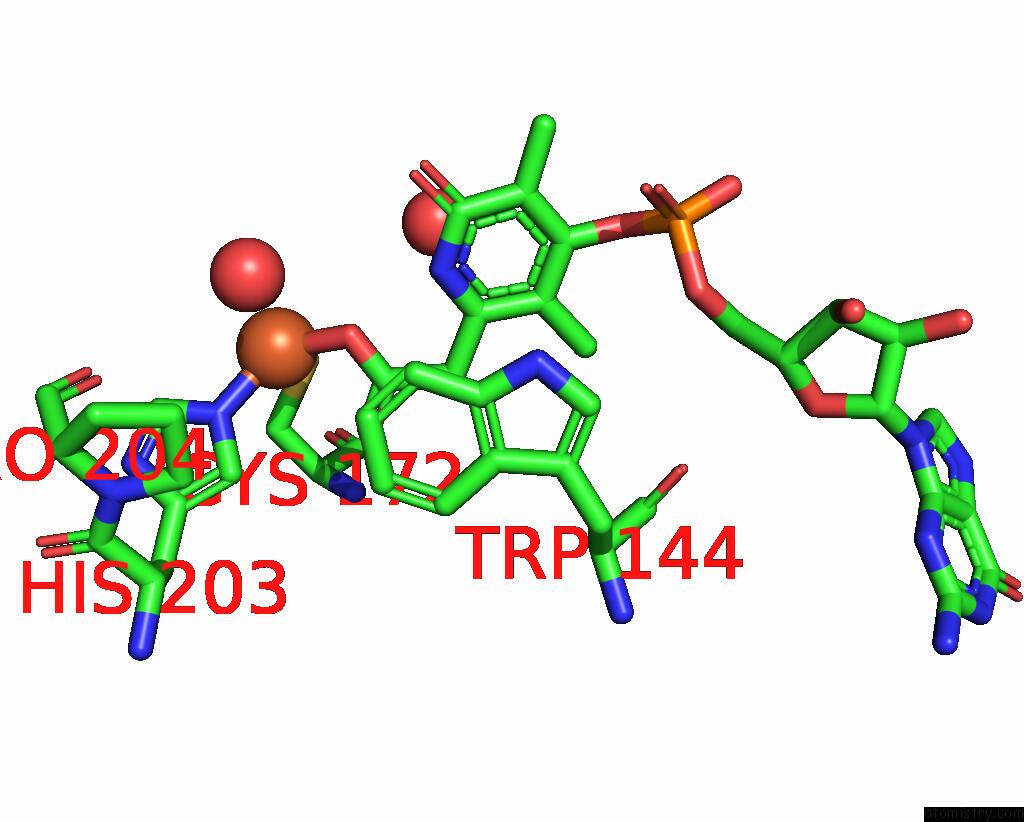
Mono view
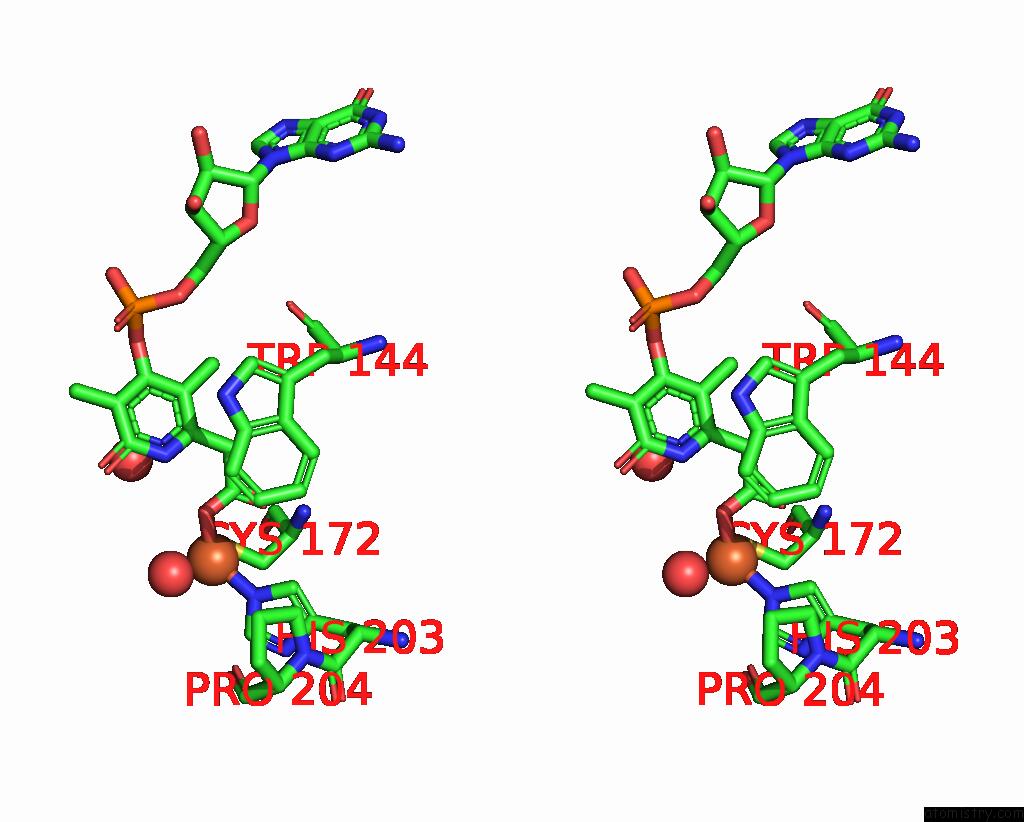
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2. within 5.0Å range:
|
Iron binding site 2 out of 3 in 5ok4
Go back to
Iron binding site 2 out
of 3 in the Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2.
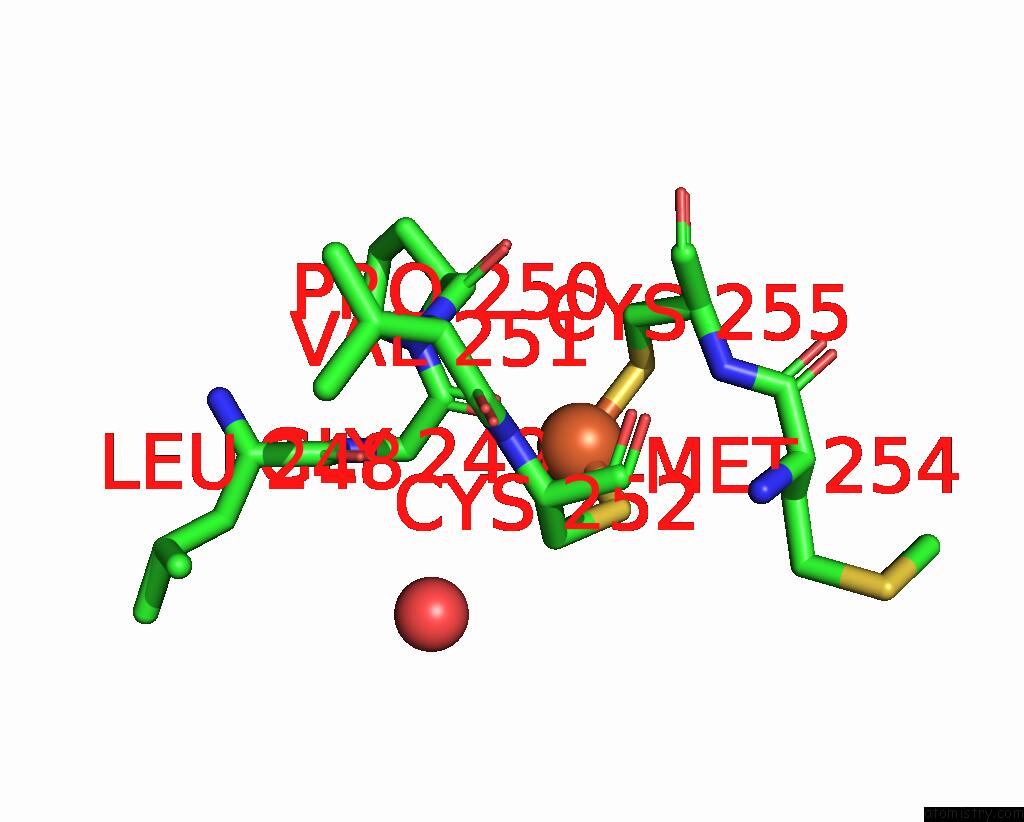
Mono view
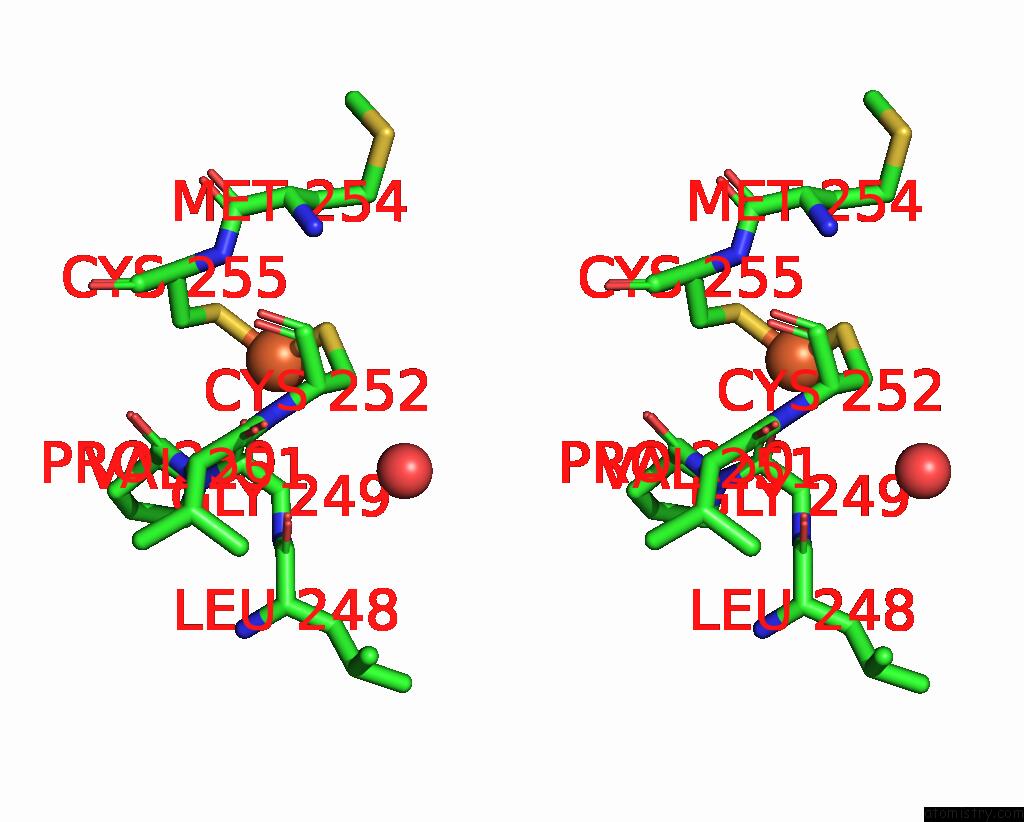
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2. within 5.0Å range:
|
Iron binding site 3 out of 3 in 5ok4
Go back to
Iron binding site 3 out
of 3 in the Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2.
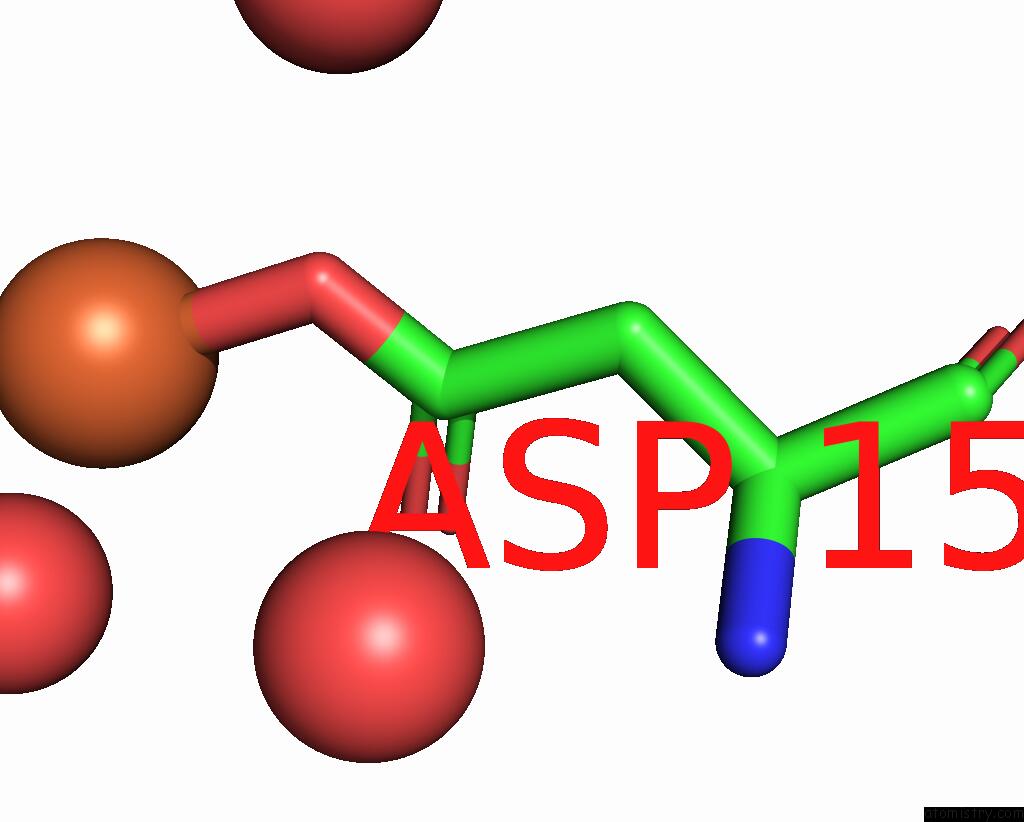
Mono view
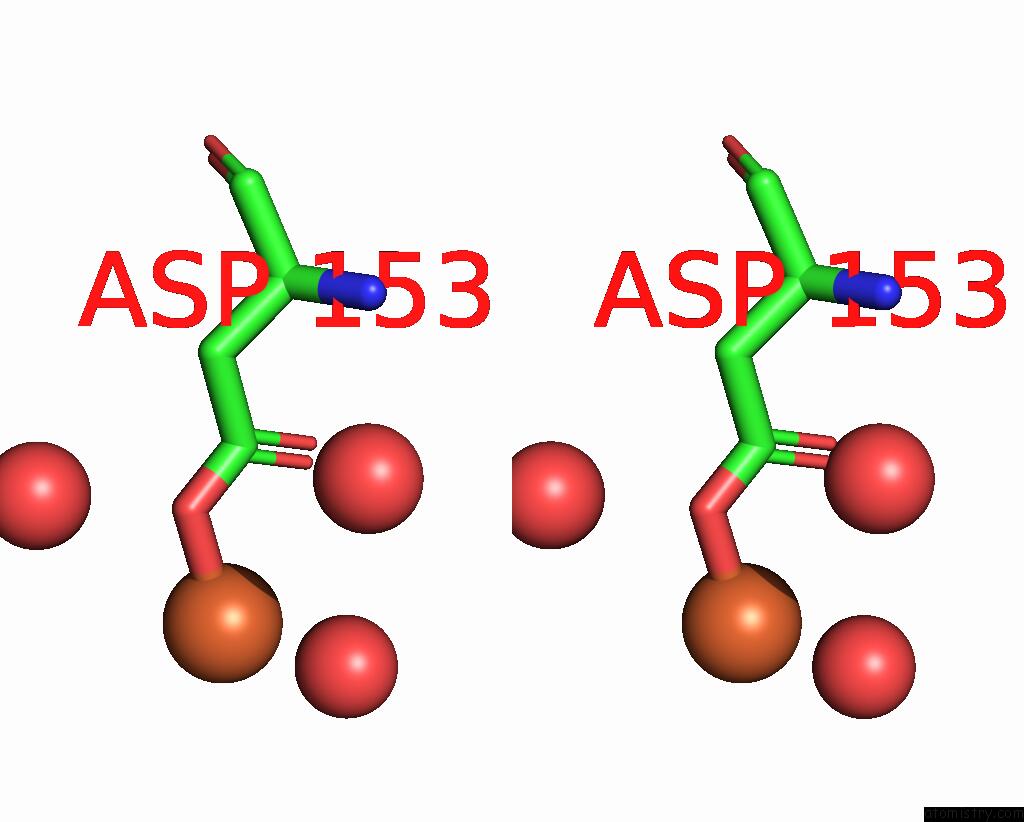
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of Native [Fe]-Hydrogenase Hmd From Methanothermobacter Marburgensis Inactivated By O2. within 5.0Å range:
|
Reference:
G.Huang,
T.Wagner,
U.Ermler,
E.Bill,
K.Ataka,
S.Shima.
Dioxygen Sensitivity of [Fe]-Hydrogenase in the Presence of Reducing Substrates. Angew. Chem. Int. Ed. Engl. V. 57 4917 2018.
ISSN: ESSN 1521-3773
PubMed: 29462510
DOI: 10.1002/ANIE.201712293
Page generated: Wed Aug 6 01:05:36 2025
ISSN: ESSN 1521-3773
PubMed: 29462510
DOI: 10.1002/ANIE.201712293
Last articles
Fe in 6ZCEFe in 6ZFU
Fe in 6ZFS
Fe in 6ZFT
Fe in 6ZAQ
Fe in 6ZAP
Fe in 6ZAN
Fe in 6ZAO
Fe in 6ZAM
Fe in 6ZAL