Iron »
PDB 6cxv-6dhz »
6d45 »
Iron in PDB 6d45: L89S Mutant of Febmb Sperm Whale Myoglobin
Protein crystallography data
The structure of L89S Mutant of Febmb Sperm Whale Myoglobin, PDB code: 6d45
was solved by
A.Bhagi-Damodaran,
E.N.Mirts,
B.Sandoval,
Y.Lu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.16 / 1.78 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 39.359, 48.075, 78.237, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.4 / 20.5 |
Iron Binding Sites:
The binding sites of Iron atom in the L89S Mutant of Febmb Sperm Whale Myoglobin
(pdb code 6d45). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the L89S Mutant of Febmb Sperm Whale Myoglobin, PDB code: 6d45:
In total only one binding site of Iron was determined in the L89S Mutant of Febmb Sperm Whale Myoglobin, PDB code: 6d45:
Iron binding site 1 out of 1 in 6d45
Go back to
Iron binding site 1 out
of 1 in the L89S Mutant of Febmb Sperm Whale Myoglobin
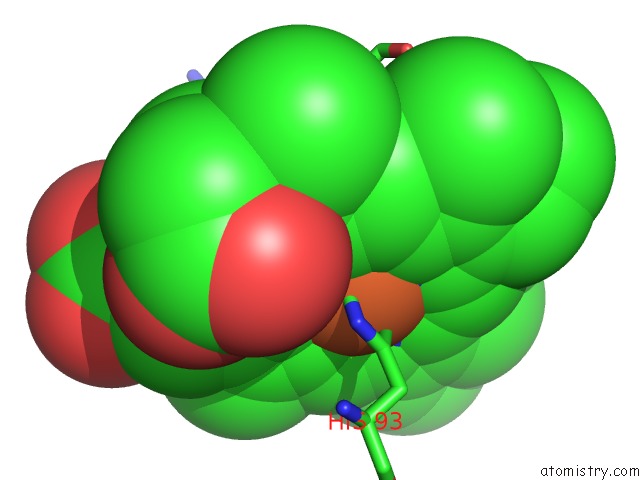
Mono view
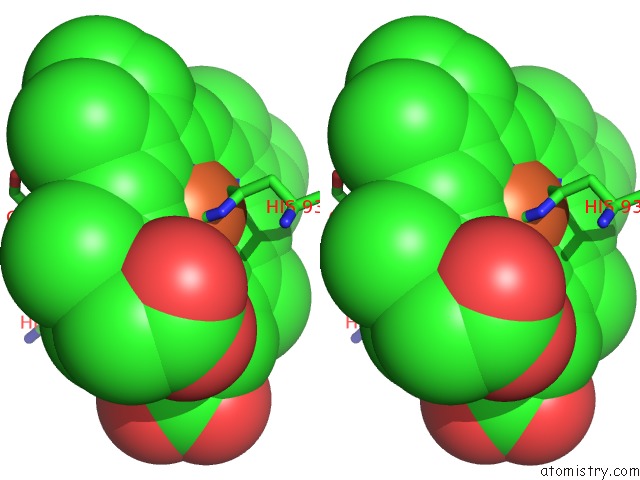
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of L89S Mutant of Febmb Sperm Whale Myoglobin within 5.0Å range:
|
Reference:
A.Bhagi-Damodaran,
J.H.Reed,
Q.Zhu,
Y.Shi,
P.Hosseinzadeh,
B.A.Sandoval,
K.A.Harnden,
S.Wang,
M.R.Sponholtz,
E.N.Mirts,
S.Dwaraknath,
Y.Zhang,
P.Moenne-Loccoz,
Y.Lu.
Heme Redox Potentials Hold the Key to Reactivity Differences Between Nitric Oxide Reductase and Heme-Copper Oxidase. Proc. Natl. Acad. Sci. V. 115 6195 2018U.S.A..
ISSN: ESSN 1091-6490
PubMed: 29802230
DOI: 10.1073/PNAS.1720298115
Page generated: Tue Aug 6 16:14:23 2024
ISSN: ESSN 1091-6490
PubMed: 29802230
DOI: 10.1073/PNAS.1720298115
Last articles
Ca in 5SBDCa in 5SBE
Ca in 5SBC
Ca in 5SBB
Ca in 5SBA
Ca in 5SB7
Ca in 5SB9
Ca in 5SB8
Ca in 5SB6
Ca in 5SB5