Iron »
PDB 6e45-6f0a »
6euo »
Iron in PDB 6euo: Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5
Protein crystallography data
The structure of Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5, PDB code: 6euo
was solved by
T.Isabet,
E.Stura,
P.Legrand,
A.Zaparucha,
K.Bastard,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 62.20 / 2.30 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 91.280, 98.930, 166.050, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.9 / 20.5 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5
(pdb code 6euo). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5, PDB code: 6euo:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5, PDB code: 6euo:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 6euo
Go back to
Iron binding site 1 out
of 4 in the Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5
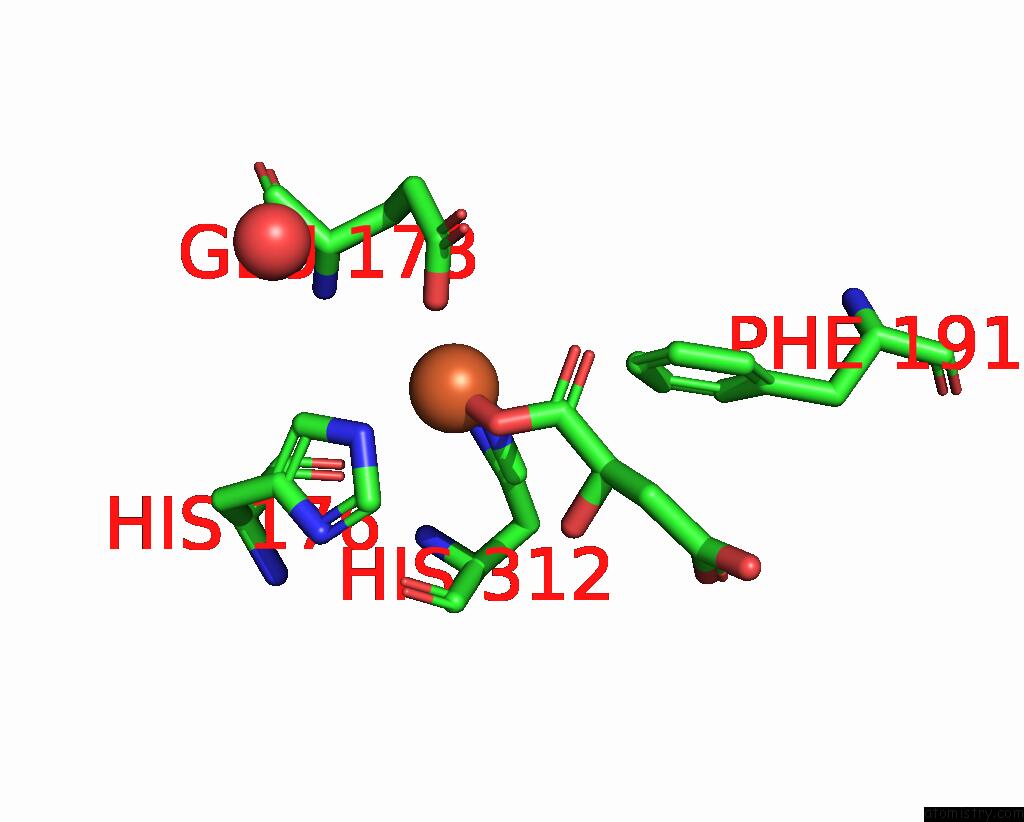
Mono view
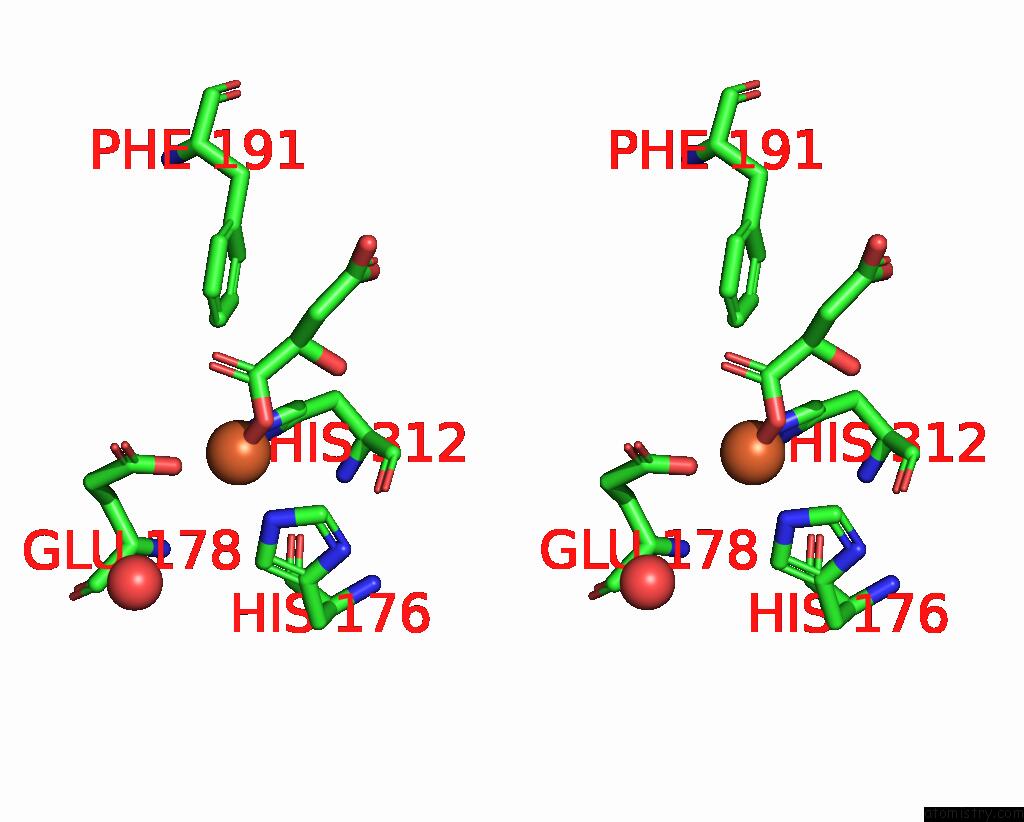
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5 within 5.0Å range:
|
Iron binding site 2 out of 4 in 6euo
Go back to
Iron binding site 2 out
of 4 in the Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5
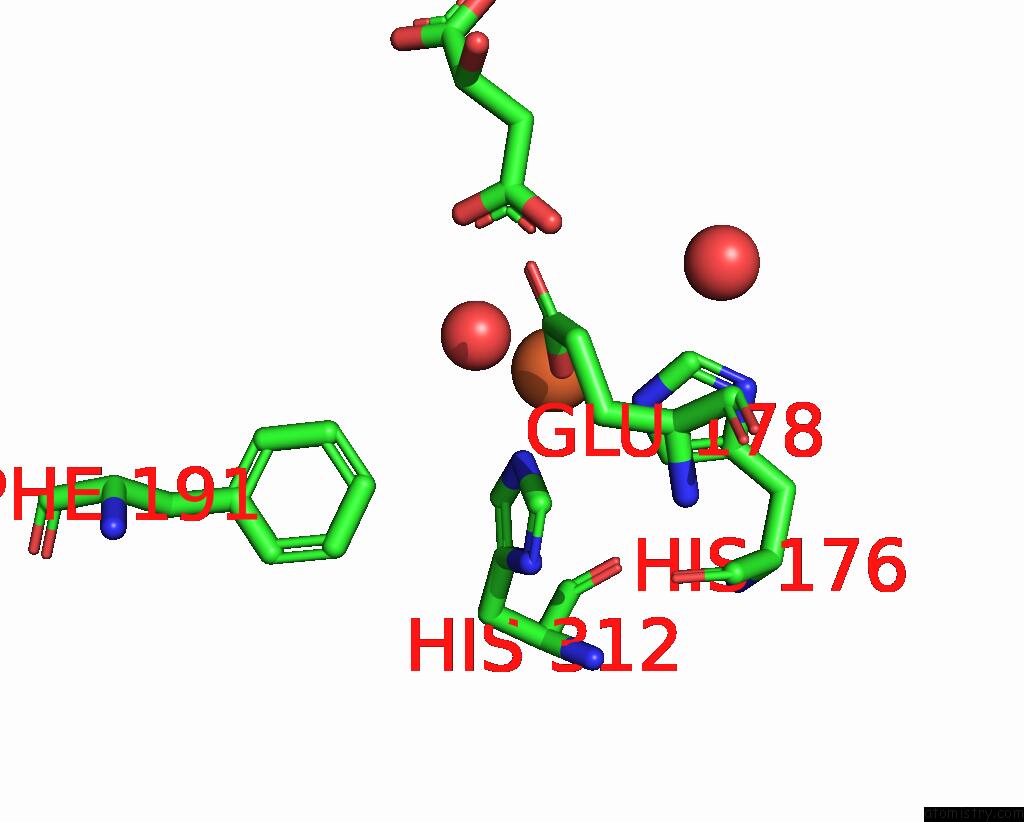
Mono view
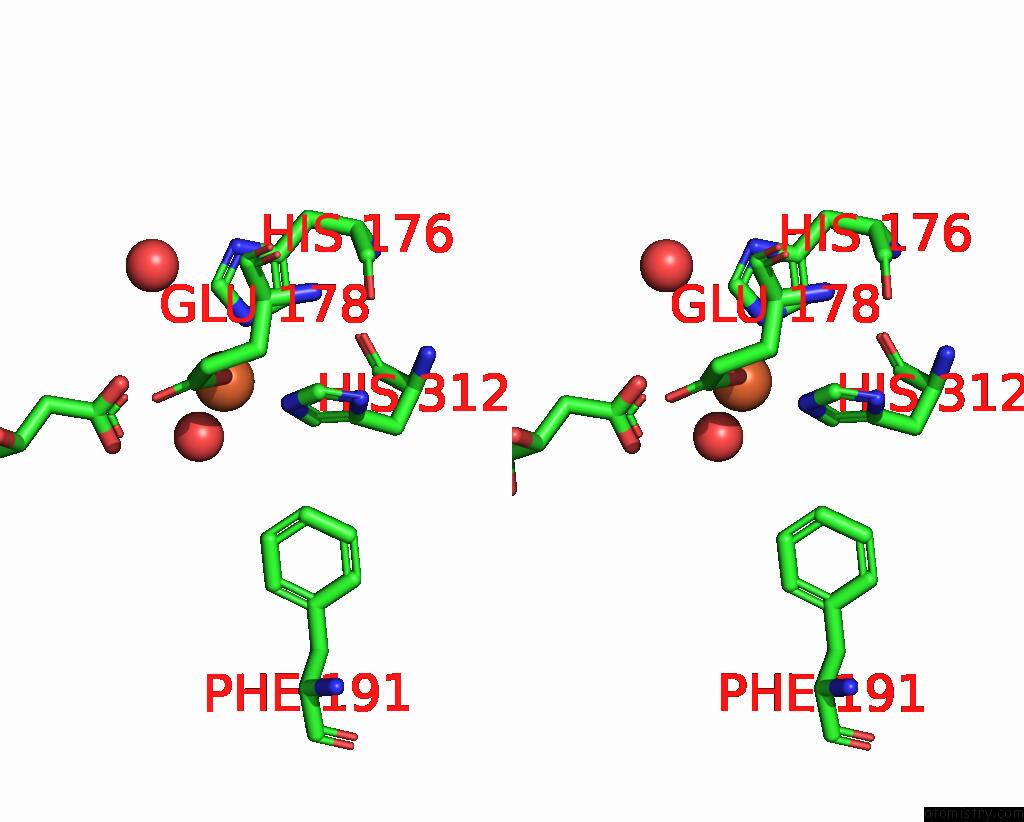
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5 within 5.0Å range:
|
Iron binding site 3 out of 4 in 6euo
Go back to
Iron binding site 3 out
of 4 in the Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5
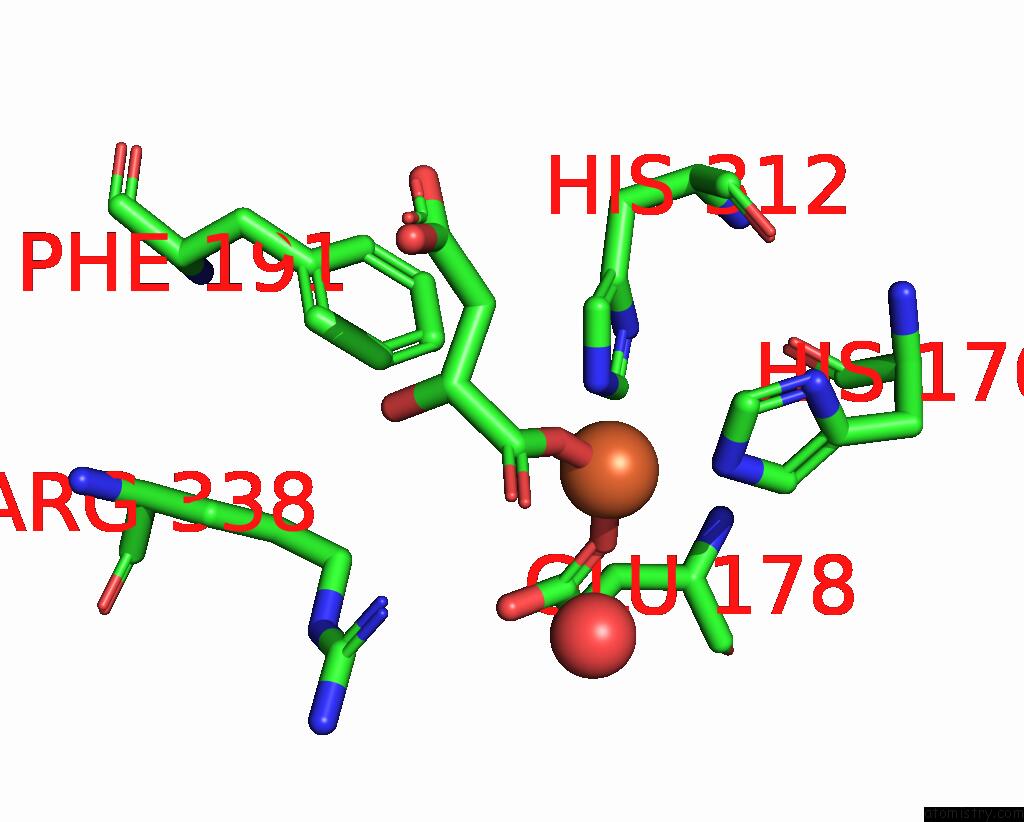
Mono view
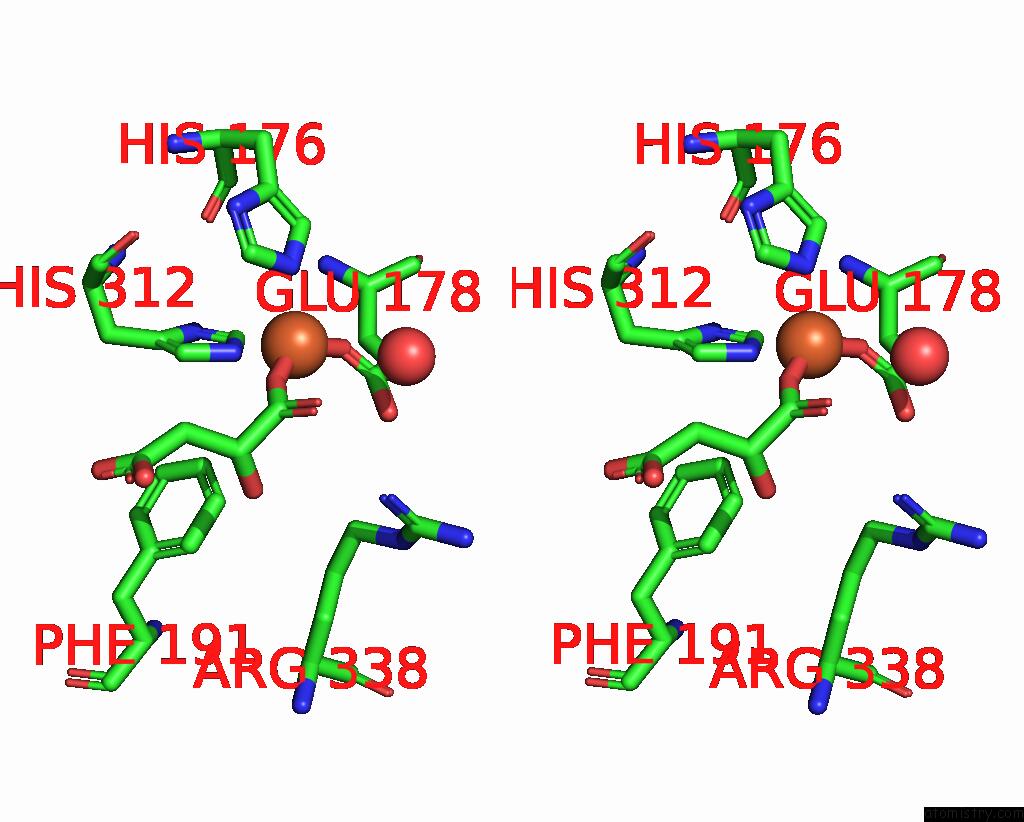
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5 within 5.0Å range:
|
Iron binding site 4 out of 4 in 6euo
Go back to
Iron binding site 4 out
of 4 in the Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5
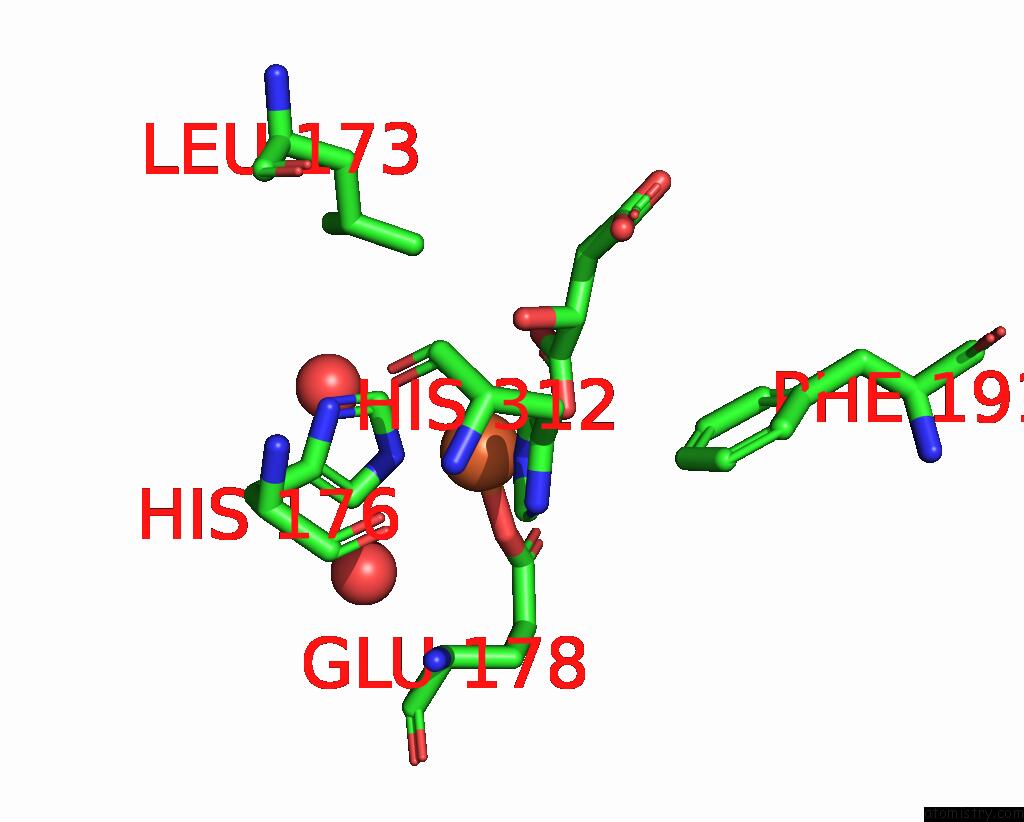
Mono view
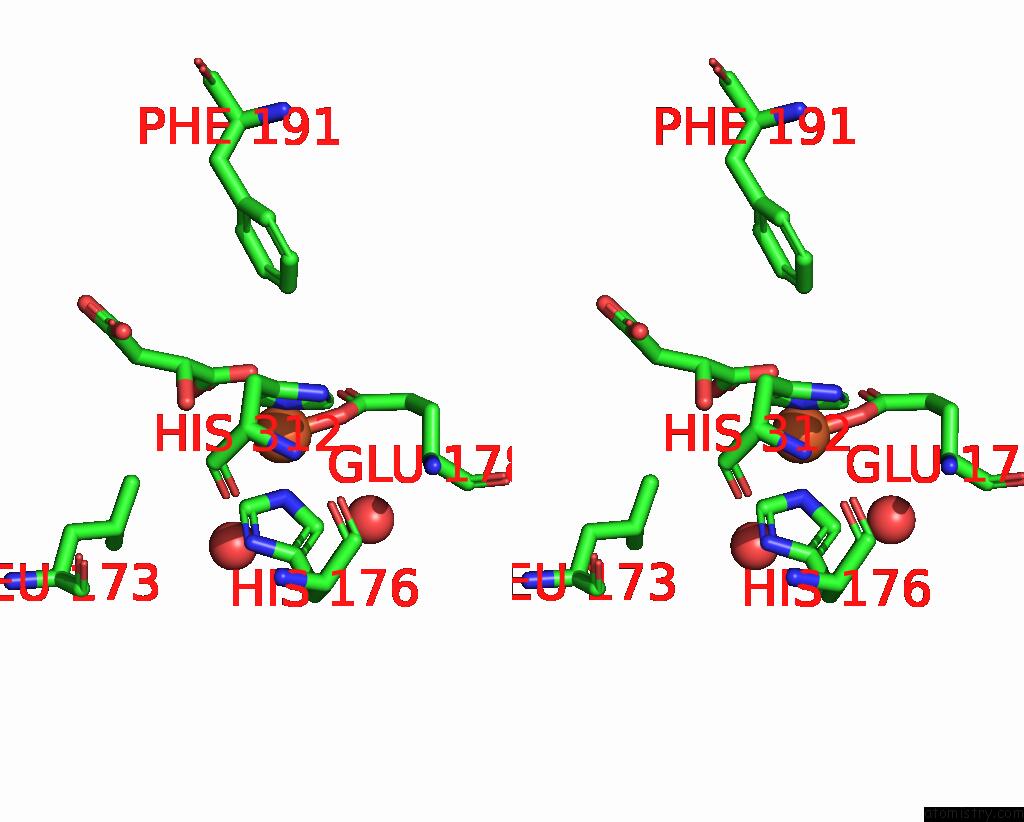
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of Apo Fe(II)/Alpha-Ketoglutarate Dependent Dioxygenase KDO5 within 5.0Å range:
|
Reference:
K.Bastard,
T.Isabet,
E.A.Stura,
P.Legrand,
A.Zaparucha.
Structural Studies Based on Two Lysine Dioxygenases with Distinct Regioselectivity Brings Insights Into Enzyme Specificity Within the Clavaminate Synthase-Like Family. Sci Rep V. 8 16587 2018.
ISSN: ESSN 2045-2322
PubMed: 30410048
DOI: 10.1038/S41598-018-34795-9
Page generated: Wed Aug 6 05:53:07 2025
ISSN: ESSN 2045-2322
PubMed: 30410048
DOI: 10.1038/S41598-018-34795-9
Last articles
Fe in 6I6GFe in 6I40
Fe in 6I43
Fe in 6I3J
Fe in 6I3T
Fe in 6I3K
Fe in 6I36
Fe in 6I2Z
Fe in 6I2J
Fe in 6I0R