Iron »
PDB 7qho-7r2s »
7r0w »
Iron in PDB 7r0w: 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
Other elements in 7r0w:
The structure of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules also contains other interesting chemical elements:
| Magnesium | (Mg) | 2 atoms |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 12;Binding sites:
The binding sites of Iron atom in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules (pdb code 7r0w). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 12 binding sites of Iron where determined in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules, PDB code: 7r0w:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 12 in 7r0w
Go back to
Iron binding site 1 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
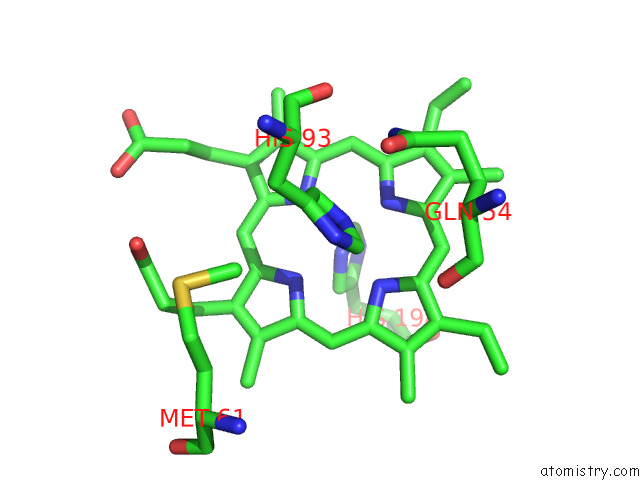
Mono view
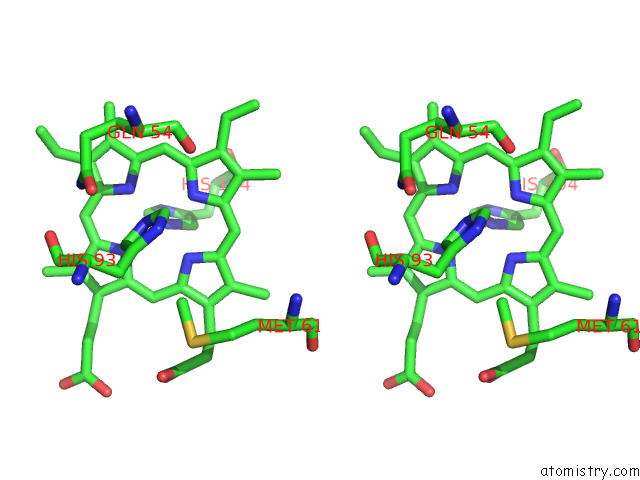
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
Iron binding site 2 out of 12 in 7r0w
Go back to
Iron binding site 2 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
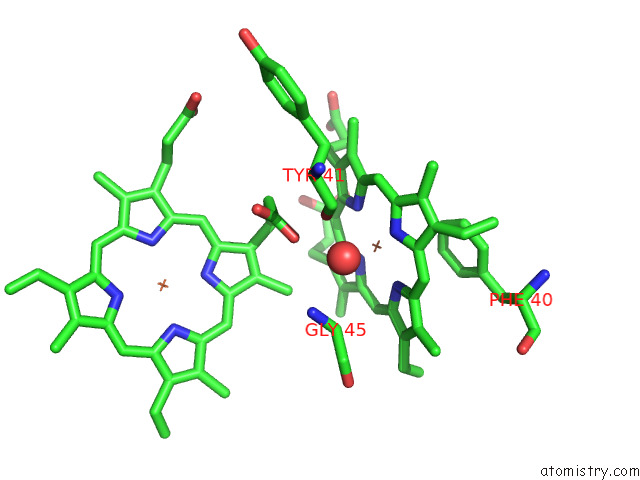
Mono view
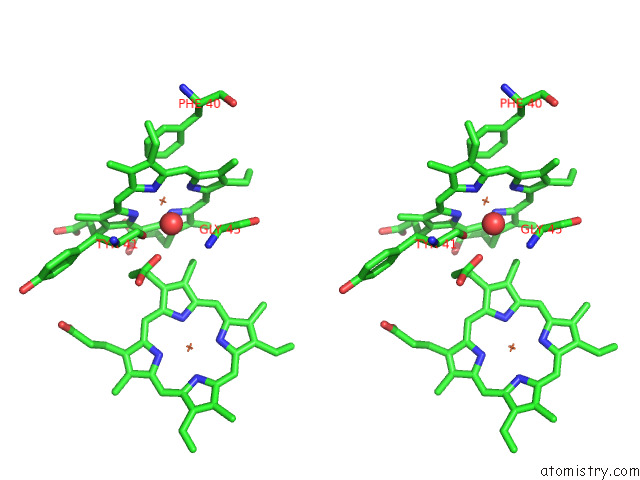
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
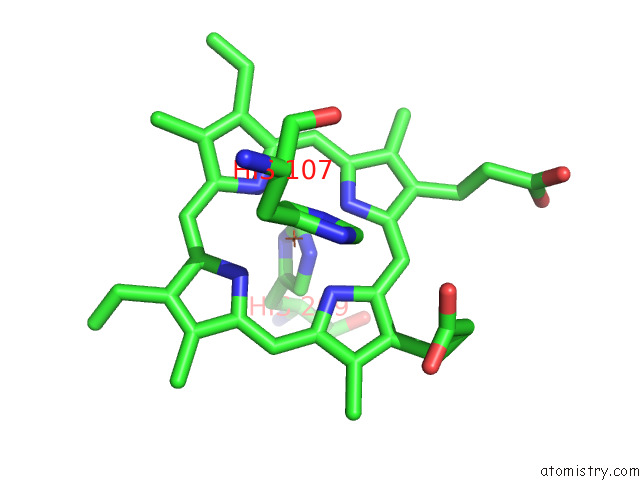
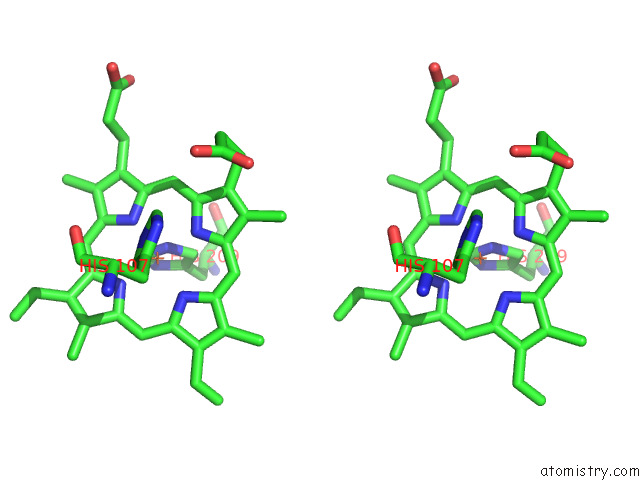
Iron binding site 3 out of 12 in 7r0w
Go back to
Iron binding site 3 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
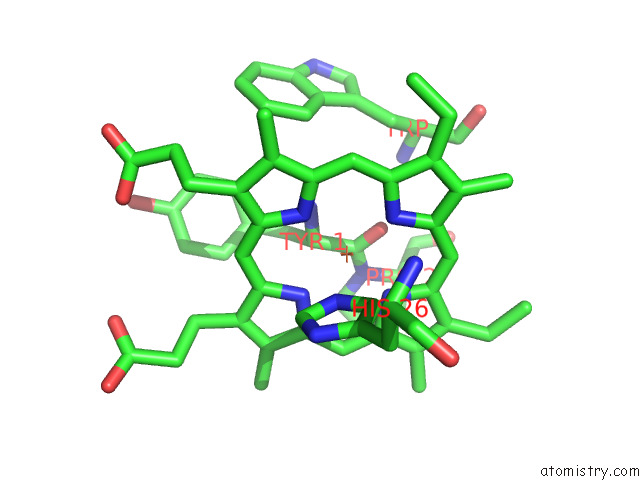
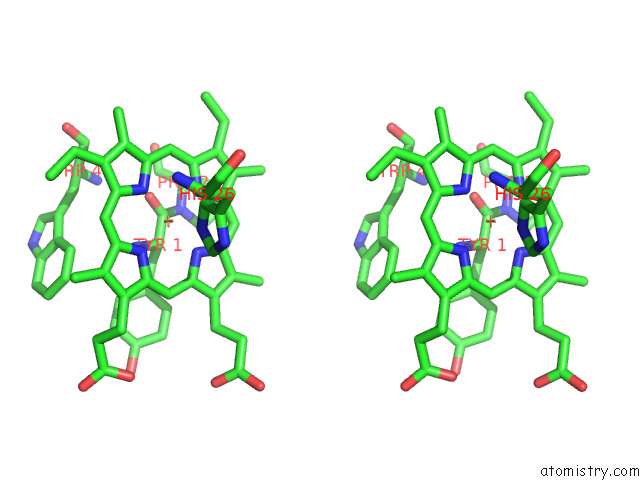
Iron binding site 4 out of 12 in 7r0w
Go back to
Iron binding site 4 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
Iron binding site 5 out of 12 in 7r0w
Go back to
Iron binding site 5 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
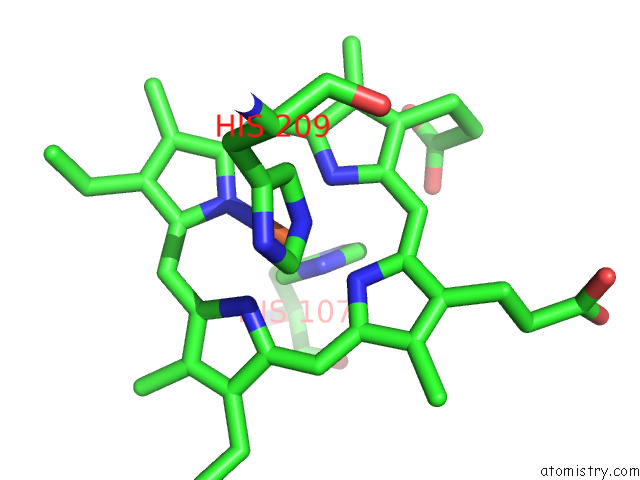
Mono view
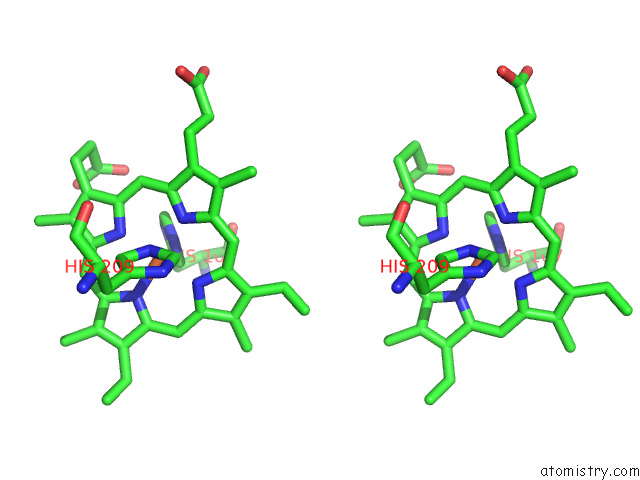
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
Iron binding site 6 out of 12 in 7r0w
Go back to
Iron binding site 6 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
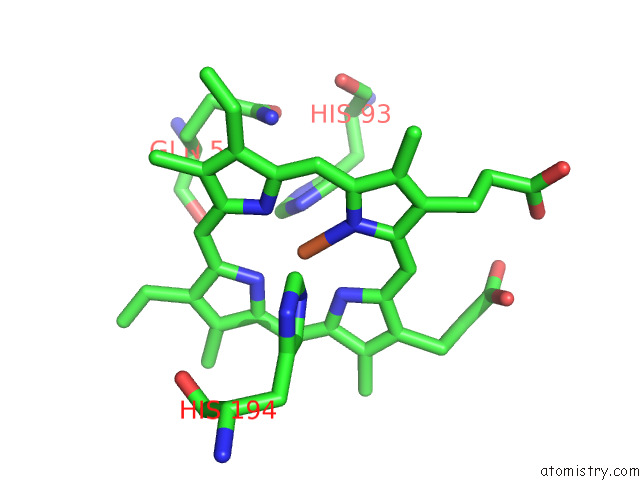
Mono view
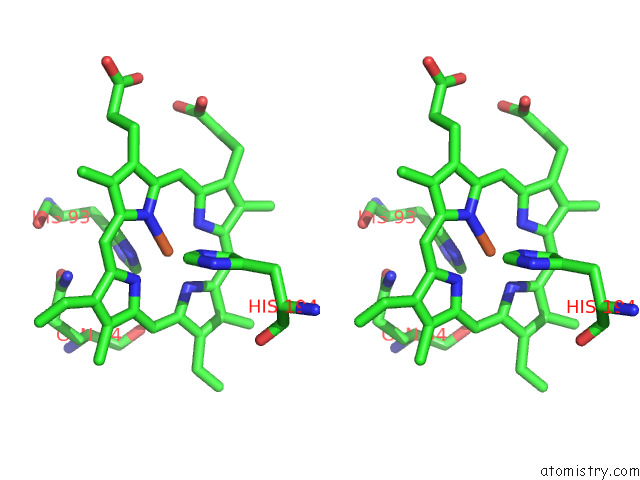
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
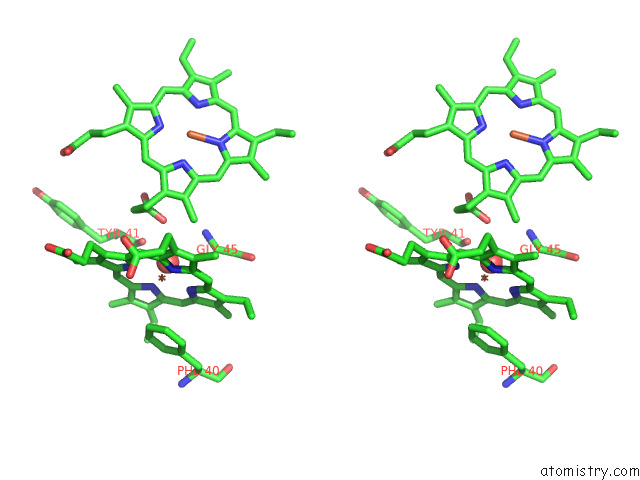
Iron binding site 7 out of 12 in 7r0w
Go back to
Iron binding site 7 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
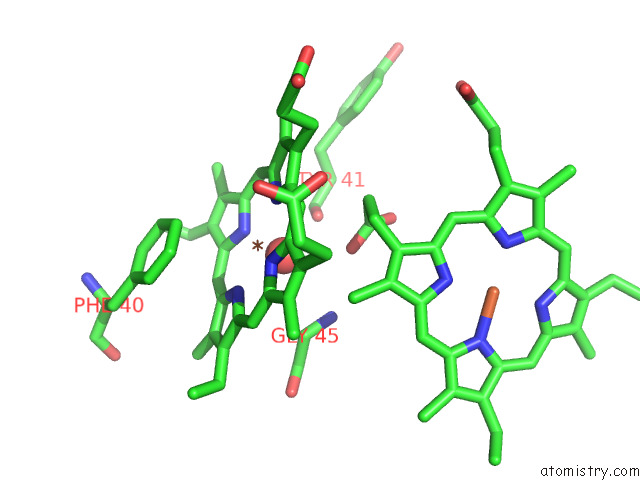
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
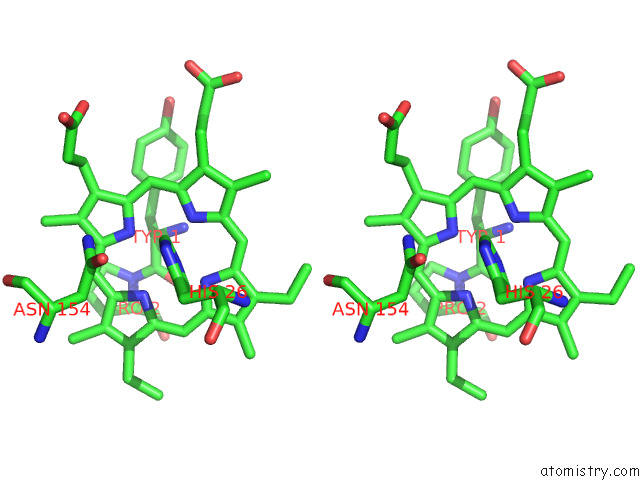
Iron binding site 8 out of 12 in 7r0w
Go back to
Iron binding site 8 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
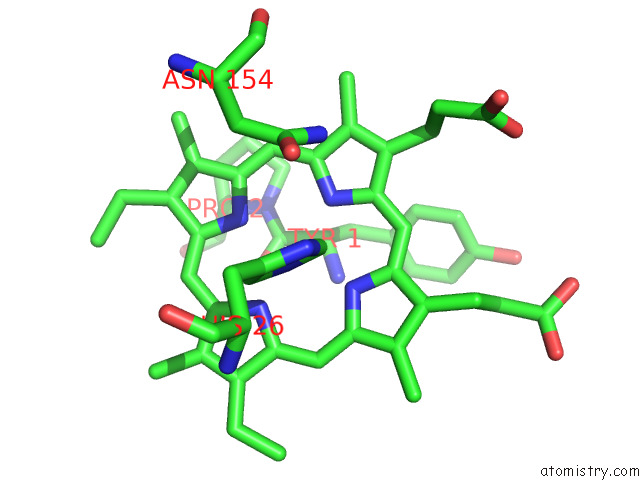
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
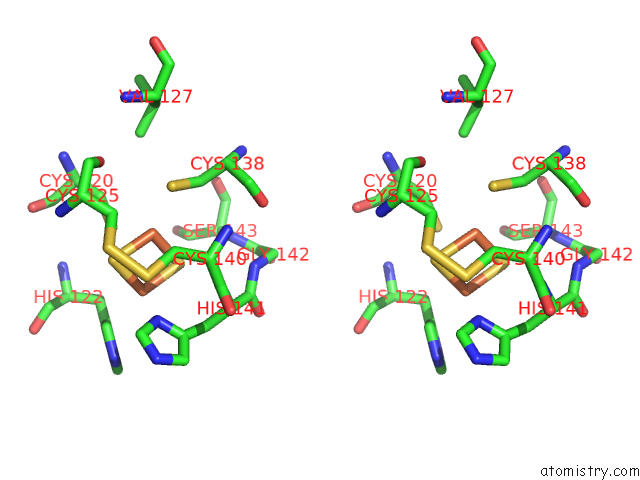
Iron binding site 9 out of 12 in 7r0w
Go back to
Iron binding site 9 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
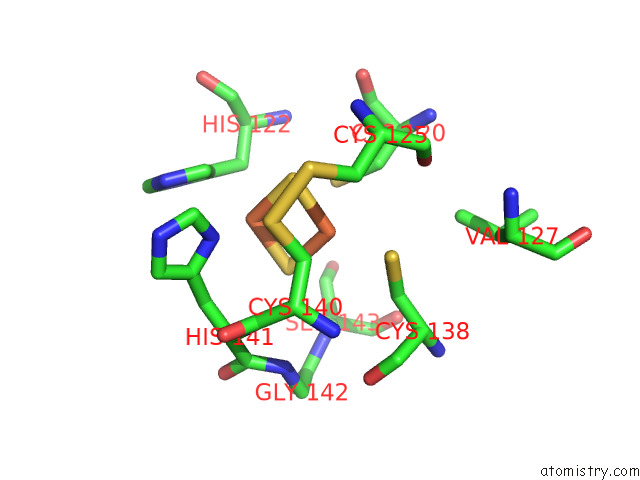
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
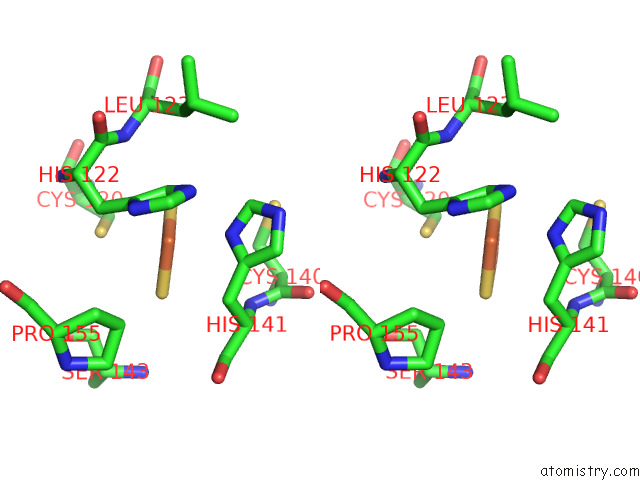
Iron binding site 10 out of 12 in 7r0w
Go back to
Iron binding site 10 out
of 12 in the 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules
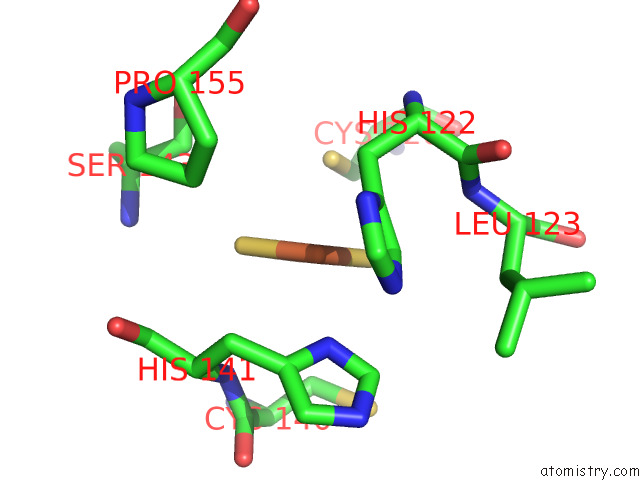
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of 2.8 Angstrom Cryo-Em Structure of the Dimeric Cytochrome B6F-Petp Complex From Synechocystis Sp. Pcc 6803 with Natively Bound Lipids and Plastoquinone Molecules within 5.0Å range:
|
Reference:
M.S.Proctor,
L.A.Malone,
D.A.Farmer,
D.J.K.Swainsbury,
F.R.Hawkings,
F.Pastorelli,
T.Z.Emrich-Mills,
C.A.Siebert,
C.N.Hunter,
M.P.Johnson,
A.Hitchcock.
Cryo-Em Structures of the Synechocystis Sp. Pcc 6803 Cytochrome B6F Complex with and Without the Regulatory Petp Subunit. Biochem.J. V. 479 1487 2022.
ISSN: ESSN 1470-8728
PubMed: 35726684
DOI: 10.1042/BCJ20220124
Page generated: Thu Aug 7 04:16:42 2025
ISSN: ESSN 1470-8728
PubMed: 35726684
DOI: 10.1042/BCJ20220124
Last articles
Fe in 7V28Fe in 7V25
Fe in 7UTA
Fe in 7UWP
Fe in 7UVB
Fe in 7UVA
Fe in 7UV9
Fe in 7UUR
Fe in 7UT9
Fe in 7UTE