Iron »
PDB 4wha-4x33 »
4wq4 »
Iron in PDB 4wq4: E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp
Enzymatic activity of E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp
All present enzymatic activity of E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp:
2.6.99.4;
2.6.99.4;
Protein crystallography data
The structure of E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp, PDB code: 4wq4
was solved by
W.Zhang,
B.Collinet,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.04 / 2.33 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 63.270, 68.070, 87.130, 109.30, 92.98, 117.23 |
| R / Rfree (%) | 20.2 / 25.3 |
Iron Binding Sites:
The binding sites of Iron atom in the E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp
(pdb code 4wq4). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp, PDB code: 4wq4:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp, PDB code: 4wq4:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 4wq4
Go back to
Iron binding site 1 out
of 2 in the E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp
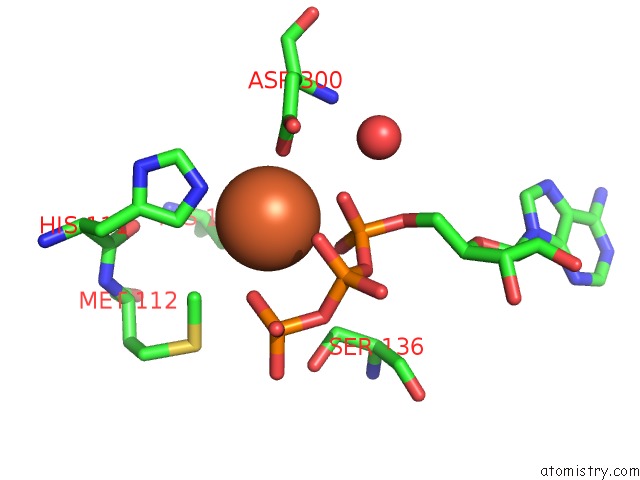
Mono view
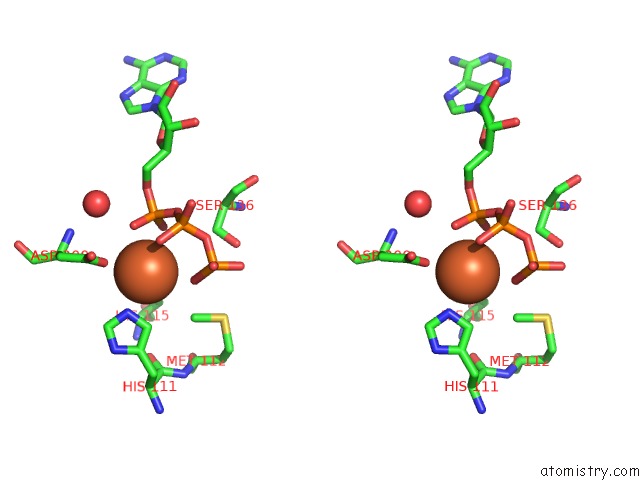
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp within 5.0Å range:
|
Iron binding site 2 out of 2 in 4wq4
Go back to
Iron binding site 2 out
of 2 in the E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp
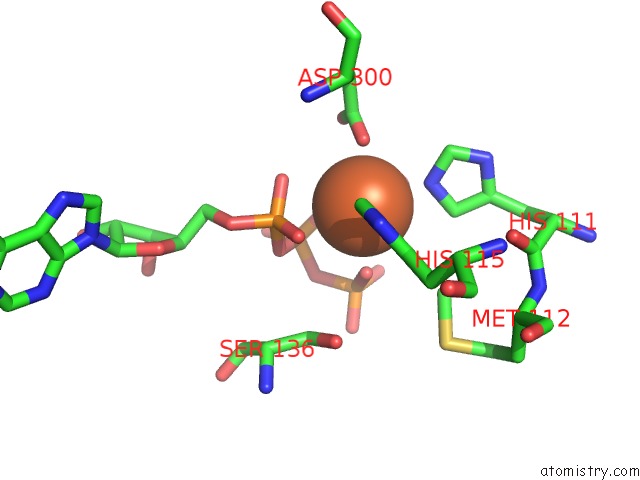
Mono view
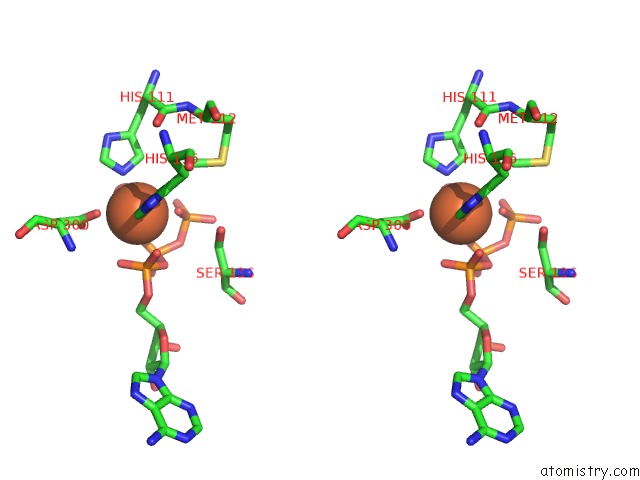
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of E. Coli Ygjd(E12A)-Yeaz Heterodimer in Complex with Atp within 5.0Å range:
|
Reference:
W.Zhang,
B.Collinet,
L.Perrochia,
D.Durand,
H.Van Tilbeurgh.
The Atp-Mediated Formation of the Ygjd-Yeaz-Yjee Complex Is Required For the Biosynthesis of Trna T6A in Escherichia Coli. Nucleic Acids Res. 2015.
ISSN: ESSN 1362-4962
PubMed: 25578970
DOI: 10.1093/NAR/GKU1397
Page generated: Tue Aug 5 16:50:39 2025
ISSN: ESSN 1362-4962
PubMed: 25578970
DOI: 10.1093/NAR/GKU1397
Last articles
Fe in 6GURFe in 6GPN
Fe in 6GM3
Fe in 6GM4
Fe in 6GPE
Fe in 6GMF
Fe in 6GM2
Fe in 6GM7
Fe in 6GM6
Fe in 6GM5