Iron »
PDB 5why-5xbp »
5wog »
Iron in PDB 5wog: Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute
Protein crystallography data
The structure of Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute, PDB code: 5wog
was solved by
R.H.Gumpper,
J.R.Terrell,
M.Luo,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.45 / 1.54 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 75.360, 82.890, 83.600, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.4 / 20.7 |
Iron Binding Sites:
The binding sites of Iron atom in the Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute
(pdb code 5wog). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute, PDB code: 5wog:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute, PDB code: 5wog:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 5wog
Go back to
Iron binding site 1 out
of 4 in the Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute
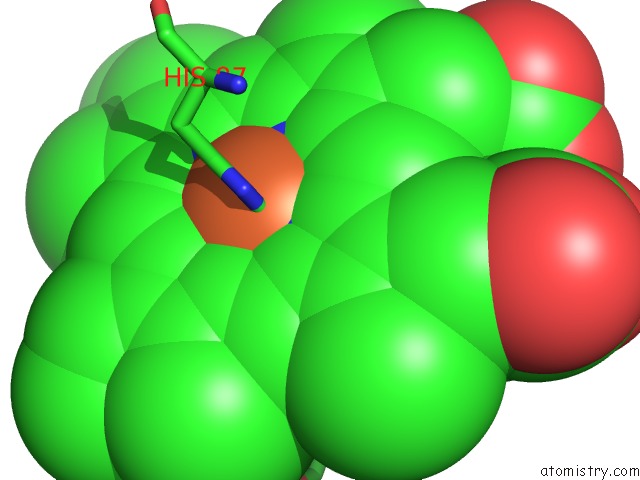
Mono view
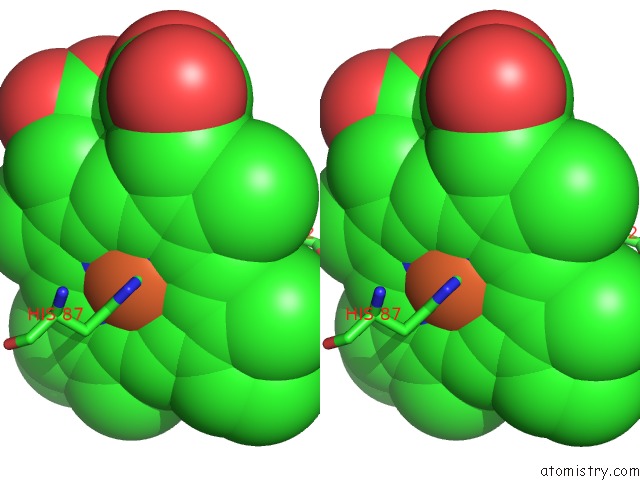
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute within 5.0Å range:
|
Iron binding site 2 out of 4 in 5wog
Go back to
Iron binding site 2 out
of 4 in the Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute
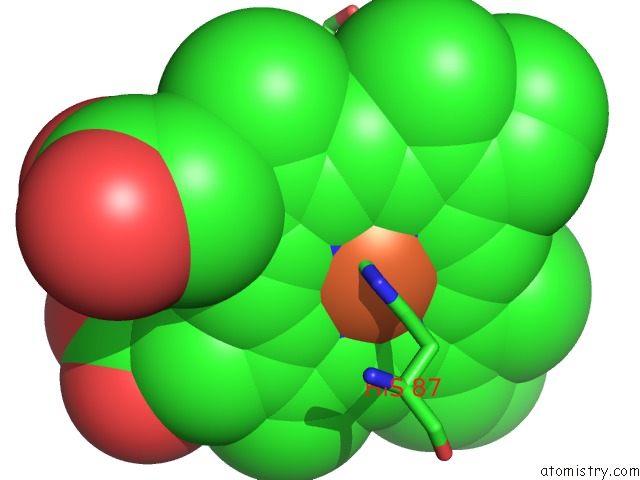
Mono view
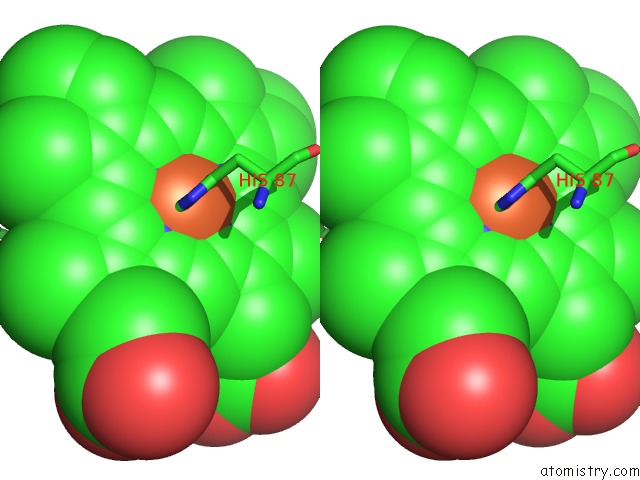
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute within 5.0Å range:
|
Iron binding site 3 out of 4 in 5wog
Go back to
Iron binding site 3 out
of 4 in the Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute
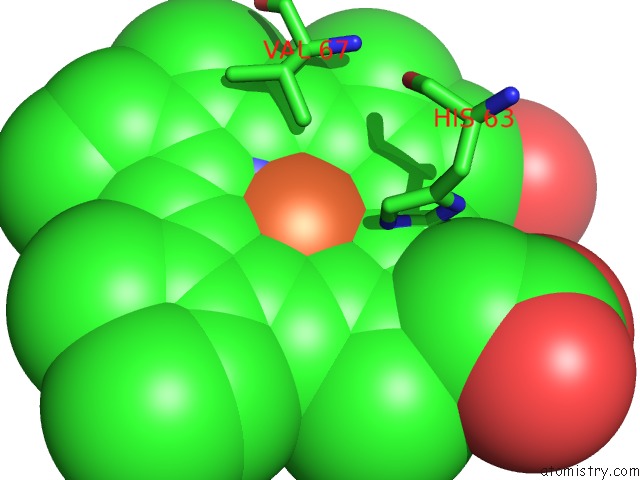
Mono view
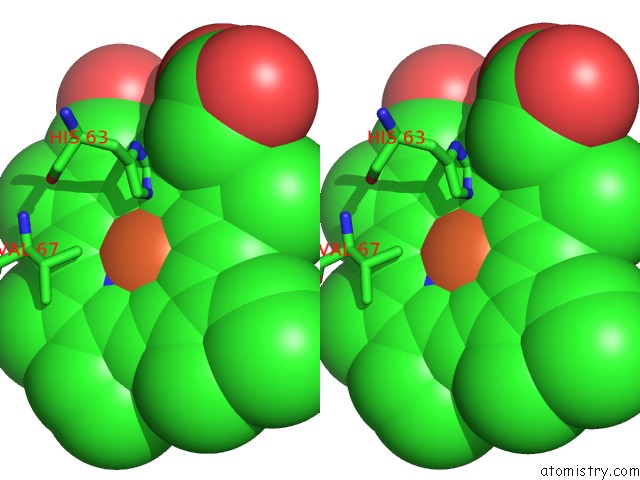
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute within 5.0Å range:
|
Iron binding site 4 out of 4 in 5wog
Go back to
Iron binding site 4 out
of 4 in the Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute
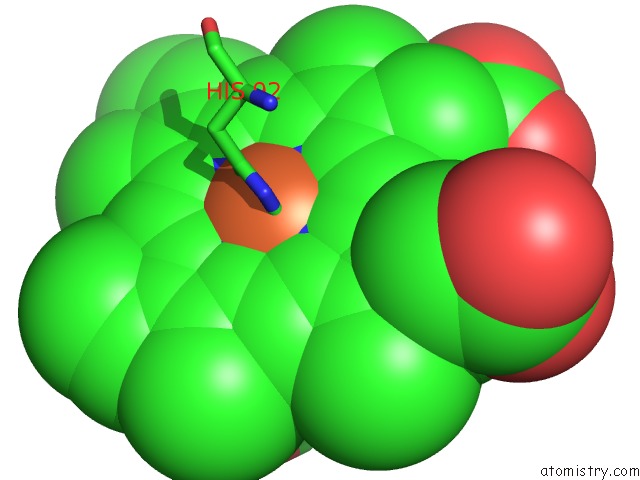
Mono view
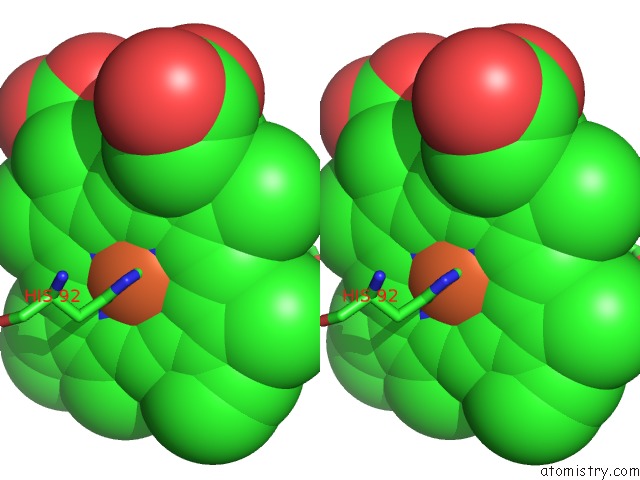
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Human Hemoglobin Immersed in Liquid Oxygen For 1 Minute within 5.0Å range:
|
Reference:
J.R.Terrell,
R.H.Gumpper,
M.Luo.
Hemoglobin Crystals Immersed in Liquid Oxygen Reveal Diffusion Channels. Biochem. Biophys. Res. V. 495 1858 2018COMMUN..
ISSN: ESSN 1090-2104
PubMed: 29246762
DOI: 10.1016/J.BBRC.2017.12.038
Page generated: Wed Aug 6 02:32:36 2025
ISSN: ESSN 1090-2104
PubMed: 29246762
DOI: 10.1016/J.BBRC.2017.12.038
Last articles
Fe in 6CXUFe in 6CSB
Fe in 6CWW
Fe in 6CVC
Fe in 6CUN
Fe in 6CTC
Fe in 6CSD
Fe in 6CUK
Fe in 6CIP
Fe in 6CIN